Reports: UR351716-UR3: Ligands for the Cobalt-Catalyzed Dimerization of Alpha Olefins
Richard D. Broene, Bowdoin College
Tethered Ligands
Building upon our successful synthesis of a Cp*quinolineCoI2
last year, we attempted to reduce the Co(III) to Co(I) in the presence of
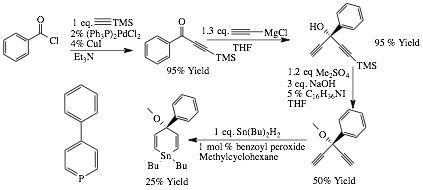
ethene to form our pre-catalyst. Scheme 1. Isocyanides. We synthesized and purified six para-substituted
phenylisocyanides that span the electronic range from -NO2 to -OMe. Scheme 2. Planar Ligands.