Reports: DNI151710-DNI1: Enantioselective Allylic Amination of Olefins
Uttam K. Tambar, PHD, University of Texas Southwestern Medical Center at Dallas
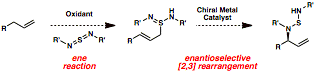
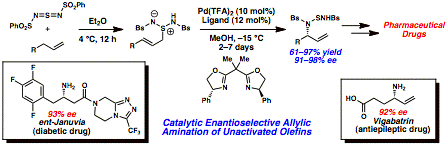
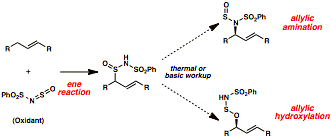
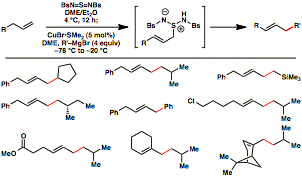
Uttam K. Tambar, PHD, University of Texas Southwestern Medical Center at Dallas





Copyright © American Chemical Society