Reports: ND451880-ND4: The Selective and Sequential Chemistry of Electronically-Coupled Lactones
Ronald K. Castellano, University of Florida
A. Background and significance.
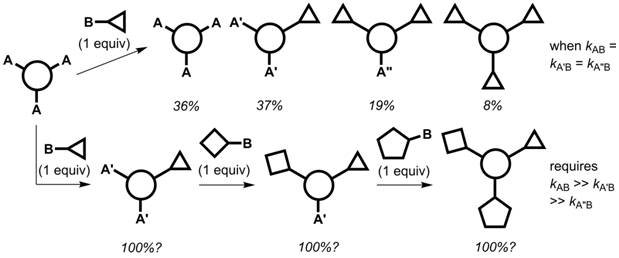
Synthetic methodology to rapidly and efficiently prepare multifunctional molecules continues to leverage discoveries in disciplines spanning materials science and chemical biology. One approach to discrete multifunctional molecules is illustrated in Figure 1. A statistical mixture (with the percentages shown) results if the reaction rates between reagent site B and positions A, A′, and A″ are identical (top pathway). If each successive functionalization reaction sufficiently deactivates the remaining reactive sites, and a reactivity gradient is established, a one-pot sequential multifunctionalization can be executed (bottom pathway).
Figure 1. The kinetics associated with statistical and non-statistical syntheses of discrete multifunctional molecules from a three-fold symmetrical precursor.
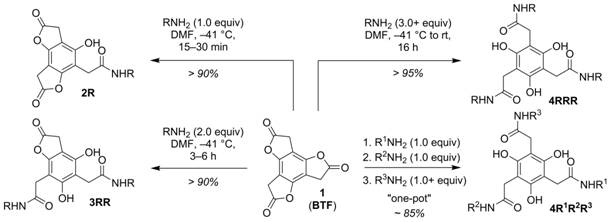
We recently reported that benzotrifuranone (BTF) 1 (Scheme 1) is uniquely suited for multifunctionalization through sequential aminolysis reactions. The platform affords rapid access to mono- (2), di- (3), and trifunctionalized (4) targets provided routine control of temperature and amine reagent stoichiometry, and lends itself to the one-pot synthesis of multifunctionalized 4 (i.e., 4R1R2R3) from 1 in excellent yield (85%) and a single day. During the last funding period we obtained a more comprehensive understanding of the aminolysis behavior of BTF (1) through comparative kinetics measurements, X-ray crystal structure analysis, and quantum chemical calculations. Particularly useful for the studies was BTF’s six-membered lactone congener, benzotripyranone (BTP). Data from the studies, the basis for an in-preparation full paper, pointed strongly to a “ring strain gradient” as the most significant selectivity driver for 1. Work this past year has sought to (a) develop a novel, efficient synthesis of 1 and (b) combine the multifunctionalization capabilities of 1 with “click chemistry” toward the preparation of functional molecular systems and materials.
Scheme 1. Aminolysis of benzotrifuranone (BTF) 1.
B. New synthetic approaches to benzotrifuranone (BTF; 1).
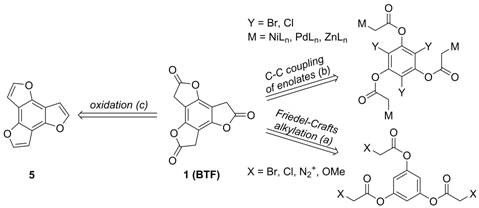
Our published approach to BTF (1) routinely affords the target in ~ 5% overall yield over four steps from commercially-available material. This past year we have explored three alternative approaches to BTF from modified phloroglucinol starting materials (Scheme 2): (a) lactone formation via intramolecular Friedel-Crafts alkylation; (b) intramolecular arylation of pendant enolates; (c) preparation and oxidation of benzotrifuran 5. Each approach is briefly discussed below.
Scheme 2. (Retro)synthetic approaches to benzotrifuranone
(BTF) 1 explored during this reporting period.
Pathway (a): Despite encouraging precedent in the
literature, attempts to realize lactone formation via
intramolecular Friedel-Crafts alkylation of
appropriately acylated phloroglucinols
have been unsuccessful. Several leaving groups were evaluated (X = Br, Cl, N2+, OMe)
in the presence of suitable activators (e.g., H+, AlCl3)
but in no cases was cyclization observed.
Pathway (b): We have looked extensively at
metal-mediated cross-coupling approaches to effect lactone
formation through the intramolecular reaction of haloarenes
and pendant enolates or enolate
equivalents. Taking a clue from Hartwig and
coworkers who reported Pd-catalyzed indolone (oxindole) formation from appropriate 2-haloanilides, we
first explored the complementary cyclization of
2-halophenylacetates. Even upon variation of the leaving group (Y = Br, Cl), the catalyst precursor (e.g., Pd2(dba)3, Pd(OAc)2),
the phosphine ligand, the
base (e.g., LiHMDS, KHMDS, LDA, n-BuLi, NaOtBu, etc.),
additives (e.g., Cy2BOTf, TMSCl), solvent,
temperature, and pre-enolization time, only deacylation to give the phenol (potentially through
hydrolytic work-up) was observed.
Suspecting that the basicity of
the enolate-generating conditions or enolates themselves are problematic in the approaches
outlined above, we are currently investigating Reformatsky
strategies (where M = NiLn or ZnLn). Work thus far has been performed
using a simpler model system, 2-bromophenyl 2-chloroacetate, where quantitative
reduction of the starting material (to afford the corresponding acetate)
suggests that metal enolate generation is occurring
efficiently. Under the best enolate generating
conditions with this substrate (Zn, rt,
1 h), a screen of various Pd sources and phosphine ligands has unfortunately not provided conversion to the
desired benzofuranone. Further optimization is
underway.
Pathway (c): Given that efficient synthetic methods
exist for the oxidation of benzofuran to benzofuranone, equivalent oxidation of benzotrifuran
(5; Scheme 2) could afford BTF. Building off of 2013 chemistry
reported by Nakamura and coworkers for accessing functionalized benzotrifurans, we have been able to access parent 5,
a novel compound, in three steps (and up to 35% yield) from
commercially-available material. Oxidation of 5 is currently being
investigated. We are separately evaluating the ability of 5 to
undergo stepwise functionalization chemistry (Figure 1) and results along these
lines will be reported in due course. In a
separate line of investigation (summarized in Scheme 3) we are evaluating the
compatibility of “click” reactions, specifically the copper-catalyzed azide-alkyne cycloaddition (CuAAC), with the multifunctionalization
opportunities afforded through aminolysis of BTF (1).
We envision the merging of the two methodologies will provide a powerful way to
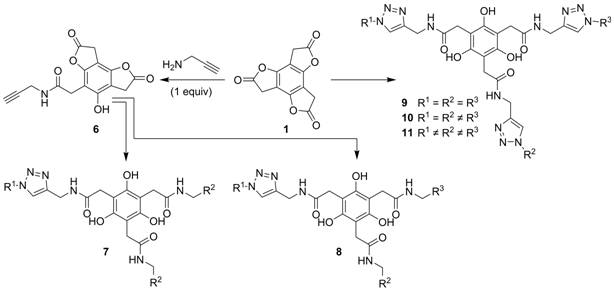
access biologically useful molecules or multifunctional materials. Scheme 3. Functionalization
of BTF (1) through iterative aminolysis and CuAAC reactions to afford multifunctionalized
architectures. BTF (1)
reacts smoothly with propargylamine to afford 6
(in > 80% isolated yield). Tested using upwards of eight different azides, standard aqueous-based CuAAC
reactions with 6 then work efficiently and without compromising the
pendant lactones. The resulting triazoles
(not shown) can be converted to 7 or 8, the latter through our
standard stepwise (but one pot) aminolysis procedure
(vide supra). Worth noting, we have found that 7 and 8 can
alternatively be prepared from 6 by reversing the order of the aminolysis and CuAAC steps.
In a similar vein, symmetrical tristriazole 9
can be prepared efficiently from 1 in two steps, recently shown in our
lab for R = hexyl. Differentiated versions 10
and 11 should be accessible through iterative aminolysis/CuAAC sequences which are currently under investigation. D. Final Remarks. Synthetic multifunctionalization
strategies are critical for realizing sophisticated properties from molecules
sufficient for their broad applications in the biological and materials
sciences. The chemistry described above, performed by two graduate
students and one undergraduate, is working towards this
goal.