Reports: UR1050070-UR10: Controlling Morphology in Bulk Heterojunction Solar Cells via Chemical Design and Surface Patterning Methods
Lee Y. Park, PhD, Williams College
Absorption profiles for polyphenylenevinylenefilms cast onto a variety of surfaces
In addition, we began investigating small molecule analogs of polyphenylene vinylenes as well, and in the later part of the period covered by the grant, this is where the bulk of our efforts have been focused. This shift in focus came about in part because of fundamental challenges that we encountered in fully characterizing the fluorinated copolymers that we had made, and in part because we realized that working with small molecule analogs would allow us much better control over the fundamental electronic properties that we were most concerned with.
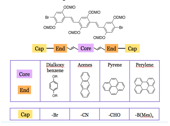
We developed a modular synthetic route to a series of oligophenylenevinylenes in which core and endgroup substituents could be varied systematically in order to study the impact of the structural variations on electronic and optical properties. We initially focused on trimeric phenylenevinylene species involving phenyl and anthryl core and terminal units, as well as a variety of electron withdrawing end-capping substituents. Initial results of this project (primarily focused on anthracene containing species with bromo and aldehyde end-capping subsitutents) were presented at the National Meeting of the American Chemical Society in San Diego as well (undergraduate coauthors indicated by asterisks): "Optical and electronic properties of discrete phenylenevinylene-based oligomers" Grace C. Babula,* Chelsea D. Boydstun,* Peter L. Clement,* Christopher J. Corbett,* Alejandro R. Gimenez,* Mindy C. Lee,*Lee Y. Park.
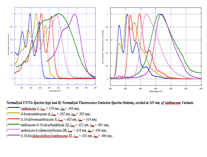
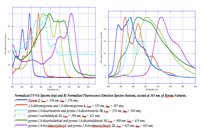
The more recent work on this project (which has extended our family to include pyrene and perylene building blocks) as well as a wider variety of end-capping groups (dimesityl boron, and cyano for instance) has been carried out by a large group of undergraduate coworkers including Chelsea Boydstun, Peter Clement, Vera Gould, Miguel Mendez, Melissa Cendejas, and Dylan Freas. We have been working to develop a more complete library of related compounds in order to systematically investigate the effect of electron withdrawing groups and various aromatic cores. We have studied the UV/Vis, fluorescence, and electrochemical behavior of our families of compounds.. We are currently working on preparing the last members of the families of compounds (extending the work to include perylene imide and diazaborolecapping groups), and hope to present the completed project at the spring ACS meeting in 2015. UV/Vis and fluorescence data are shown below.