Reports: DNI152782-DNI1: Direct Access of Chemically Differentiated 1,2-Diols from Oxime-Masked Mono-Alcohols
Guangbin Dong, PhD, University of Texas at Austin

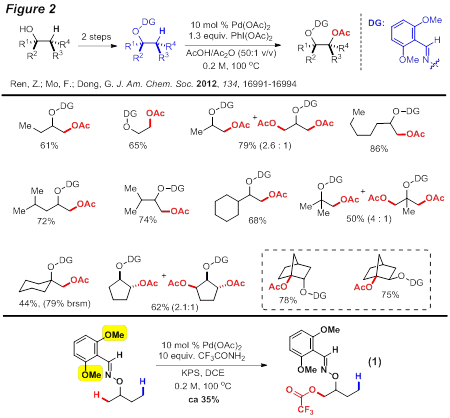
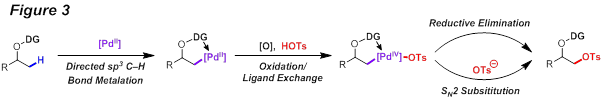
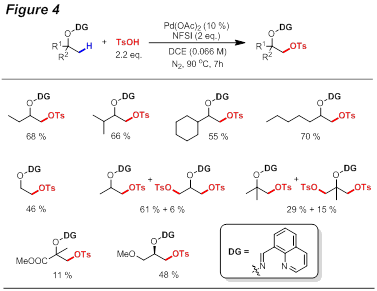
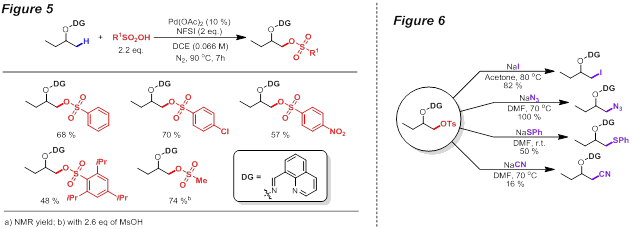
Guangbin Dong, PhD, University of Texas at Austin





Copyright © American Chemical Society