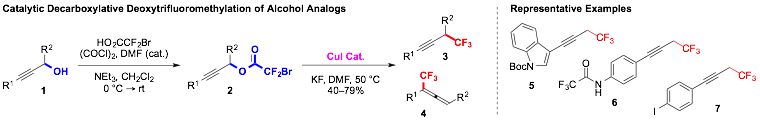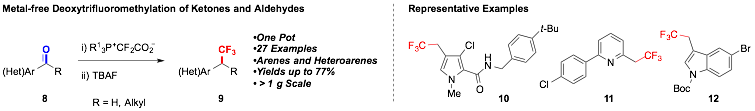Reports: DNI152073-DNI1: Development of Novel, Stable and User-Friendly Trifluoromethylation Reagents
Ryan A. Altman, PhD, University of Kansas
In the last year, PRF DNI-supported research has focused on the conversion of alcohols, aldehydes and ketones into trifluoromethyl analogs. These strategies allow for the preparation of new fluorinated compounds from alcohol- and carbonyl-containing petroleum reformats.
Fluorinated functional groups, including the trifluoromethyl group (CF3), serve important roles in medicinal, agrochemical and materials chemistry by perturbing the (bio)physical properties of the parent molecule to which the CF3 group is attached. Thus, the development of new chemicals methods to install fluorinated functional groups is critical for accessing new therapeutics, biological probes, agrochemical agents, and materials. Although many creative and useful metal-catalyzed trifluoromethylation reactions have been developed, certain simple and desired transformations have not been achieved. One such gap involves the conversion of readily available alcohol and carbonyl-based functional groups into trifluoromethyl analogs.
The general goal for the current project involved the development of new, useful and mild reagents and methods for trifluoromethylation reactions. Specifically, the proposed work identified the conversion of common functional groups (e.g. alcohols, ketones and carboxylic acids) into trifluoromethanes as a problematic synthetic sequence that required a minimum of 3–4 synthetic steps, which consumed excessive time, generated excessive waste, provided low yields of products, and used expensive and uneconomical reagents. To accomplish this goal, research activities aimed to overcome previous limitations in the field by: 1) developing short and direct strategies for converting readily available functional groups into trifluoromethyl analogs; 2) employing mild, inexpensive and environmentally-friendly reagents; 3) developing robust catalytic methods to exploit stoichiometric quantities of reagents. To overcome these limitations, the work supported by the PRF DNI award has involved the development of one- and two-step strategies for converting alcohols, aldehydes and ketones into trifluoromethyl-based analogs.
Cu-Catalyzed Trifluoromethylation of Propargylic Esters
The trifluoromethylation of propargylic alcohols (1) was accomplished in a two-step sequence involving conversion of the alcohols to bromodifluoroacetate esters (2) followed by trifluoromethylation using a catalyst system based on CuI and DMEDA. The reaction generated a wide variety of propargylic products (3) bearing useful functional groups, including aryl OMe, C(O)Me, CO2Me, NHC(O)CF3, NO2, CF3, Cl, indole. Beneficial aspects of these reactions include: 1) development of a shortened reaction sequences for converting readily available allylic alcohols into trifluoromethyl analogs (4 vs. 2 steps); 2) the ability to conduct trifluoromethylation reactions using only a catalytic quantity of metal; 3) employment of less expensive and more atom-economical source of CF3?in near-stoichiometric quantities, instead of more expensive and less atom-economical reagents common reagents in superstoichiometric quantities. However, the transformation provided a mixture of propargyl (3) and allenyl (4) products (typically > 2:1 selectivity), and ongoing work aims to optimize selectivity for accessing each product. In addition, ongoing work aims to develop analogous catalytic procedures for accessing trifluoromethanes from readily available benzylic alcohols.
Metal-Free Trifluoromethylation of Aromatic and Heteroaromatic Aldehydes and Ketones
The ability to convert simple and common substrates into fluoroalkyl derivatives under mild conditions remains an important goal for medicinal and agricultural chemists. One desirable transformation that was originally proposed involves the direct trifluoromethylation of aromatic and heteroaromatic ketones and aldehydes. The traditional approach for this net transformation involves stoichiometric metals and/or multistep reaction sequences that consume excessive time, material and labor resources while providing low yields of products. To complement these strategies, we developed a one pot metal-free decarboxylative procedure for accessing β,β,β-trifluoroethylarenes and -heteroarenes (9) from readily available ketones and aldehydes (8). This method featured several benefits, including ease of operation, readily available reagents, mild reaction conditions, high functional-group compatibility, and scalability. This reaction should provide access to a wide variety of trifluoromethylated compounds that would otherwise be challenging to access using ketone and aldehyde-based petroleum reformats as substrates.
Impact of PRF DNI Award
The PRF DNI award has positively affected Dr. Altman's career, as well as multiple group members involved in the project. This award has provided training for postdoctoral research assistants and graduate students in the fields of synthetic organic, organofluorine, and organometallic chemistries. Specifically, the researchers were intimately involved in developing the chemical methods and conducting synthetic chemistry to prepare substrates. Most notably, the researchers were challenged to think critically to solve chemical problems, and to probe the mechanistic underpinnings of the transformations. The support provided by the PRF award has positively impacted the research group by providing an opportunity to pursue basic chemical research. Further, the results obtained from PRF support will continue to drive an area of research in Dr. Altman's group for years to come, which will provide valuable synthetic tools for accessing new fluorinated analogs of compounds directly from petroleum reformats. The results described in this report provide a solid foundation for ongoing and future work in the group that aims to develop innovative decarboxylative fluorination reactions for accessing fluorinated compounds that have practical applications within many chemical sub disciplines.