Reports: ND1052674-ND10: Volatile Heterometallic Molecular Precursors for Oxide, Fluoride, and Silicate Cathode Materials of Thin Film Lithium Ion Batteries
Evgeny V. Dikarev, State University of New York at Albany
Heterometallic Precursors for the LiCoO2 Cathode Material: Design of Single-Source Precursors with Discrete Molecular Structure
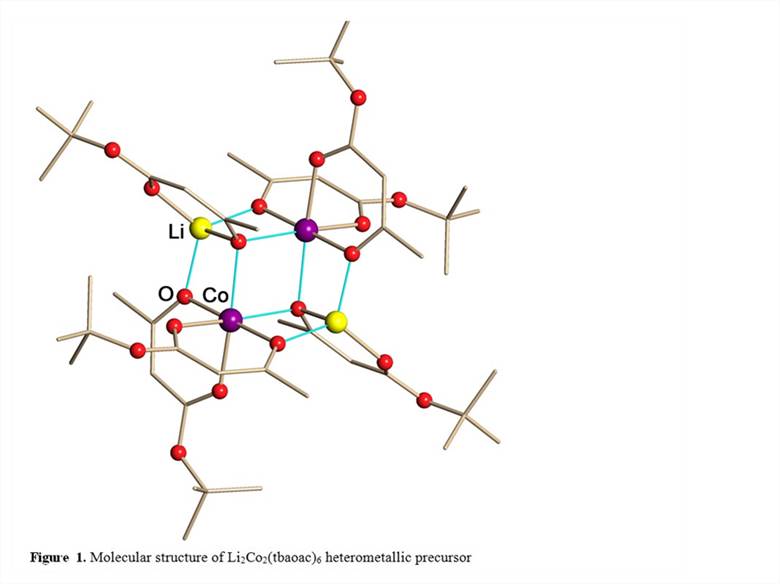
Three heterometallic single-source precursors with a proper Li:Co = 1:1 ratio for the lithium ion battery cathode material LiCoO2 have been prepared. Heterometallic compounds LiCoL3 (L = acac, tbaoac, and dhd) are volatile and can be obtained by a simple reaction procedure that employs commercially/readily available starting reagents, on a high scale with nearly quantitative yield. The first of those precursors, heterometallic diketonate LiCo(acac)3 (acac = pentane-2,4-dionate), was found to have a polymeric structure in the solid state that limits its volatility and prevents solubility in non-coordinating solvents. In order to design single-source precursors with discrete molecular structure, we have demonstrated an applicability of a novel approach by using asymmetric ligands that allow one to change the bridging connectivity pattern within heterometallic assembly. The asymmetric ligands, tert-butyl acetoacetate (tbaoac) and 2,2-dimethyl-hexanedionate (dhd) exhibit different bridging properties at the two ends of the ligand: only one of the ligand oxygens is participating in bridging of metal atoms, while the other one, adjacent to the carbon atom with bulky substituent, is simply chelating. As a result, the heterometallic complexes LiCo(tbaoac)3 and LiCo(dhd)3 that consist of discrete tetranuclear molecules Li2Co2L6 (Figure 1) have been successfully isolated and characterized. In the solid state structures of these compounds the bulky tBu and OtBu ends of the ligands are facing outward of heterometallic assembly thus providing an additional screening protection as well as preventing intermolecular bridging contacts and the formation of polymeric structures.
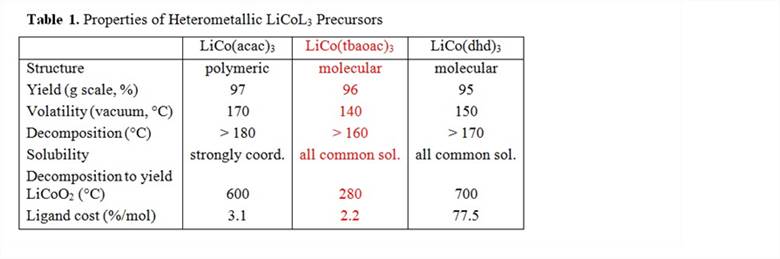
Out of three heterometallic compounds synthesized in this work, LiCo(tbaoac)3appears to meet the application demands for an ideal single-source precursor (Table 1).
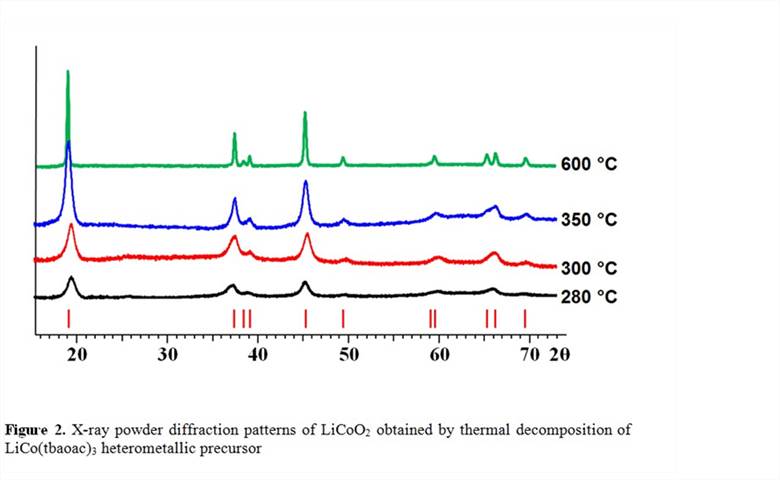
In accord with its discrete molecular structure, the complex was found to be soluble in nearly all common solvents and was shown to retain its heterometallic structure in solution. Thus, it can be effectively used for the preparation of thin films by direct liquid injection MOCVD technique. The compound is highly volatile and exhibits a congruent sublimation with heterometallic fragments present in the gas phase. In addition, it shows the lowest decomposition temperature leading to a phase-pure LiCoO2oxide (Figure 2) and can be utilized for the preparation of nanoparticles of this cathode material. Importantly, this single-source precursor has the lowest preparation costs, specifically it does not impose a high extra ligand cost on the isolation of target material – the issue that is often associated with the application of single-source precursors.
Volatile Heterometallic Precursors for the Low-Temperature Synthesis of Prospective Sodium Ion Battery Fluoride Cathode Materials
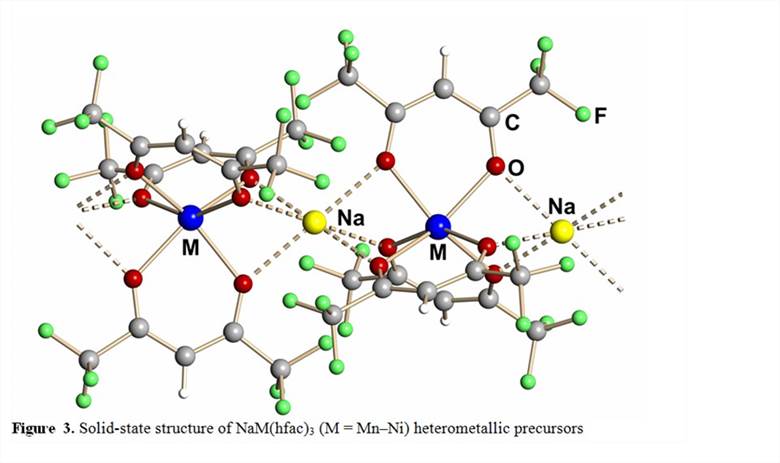
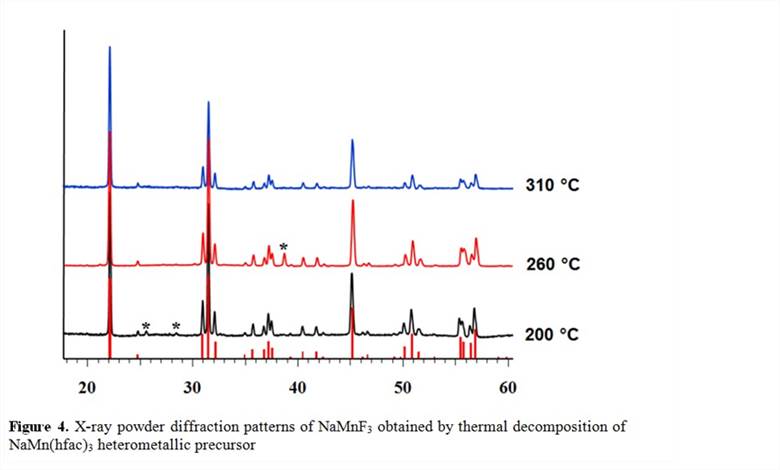
Heterometallic single-source precursors with a proper sodium:transition metal ratio for prospective non-oxide sodium ion battery cathode materials NaMF3 were developed. Heterometallic fluorinated β-diketonates NaM(hfac)3(Figure 3; M = Mn, Fe, Co, and Ni; hfac = hexafluoroacetylacetonate) have been obtained on a large scale, in high yield using one-step reaction that employs commercially available reagents. The complexes are stable in moist air and are highly volatile.
The mass spectrometric investigation indicated the existence of heterometallic molecules in the gas phase. Heterometallic precursors can be utilized in the formation of thin films of NaMF3 cathode materials by chemical vapor deposition (CVD). The presence of heterometallic species in solutions of several solvents has been unambiguously confirmed by multinuclear NMR, mass-spectrometry, as well as by isolation of tetranuclear molecules [M2Na2(hfac)6(sol)] from solution. The presence of heterometallic species in solution opens broad opportunities for application of the title precursors in direct liquid injection CVD techniques. Heterometallic precursors were shown to exhibit clean, low-temperature decomposition in argon atmosphere that results in phase-pure perovskite fluorides NaMF3(Figure 4), the prospective cathode materials for sodium ion batteries. Volatility and low-temperature decomposition properties of heterometallic diketonates were successfully utilized in the preparation of target fluoride materials in the form of thin films, nanodots, and nanorods.
Future Work
The work in the next reporting period will be focused on the three major goals: (a) design of heterometallic precursors for the preparation of lithium-iron phosphate cathode material LiFe(PO4); (b) exploration of molecular precursors for the low-temperature synthesis of new prospective cathode materials that cannot be prepared by conventional solid-state synthetic techniques; (c) development of innovative approaches to the synthesis of heterotrimetallic precursors for multimetallic oxide materials such as LiMnMO4 (M = Co, Ni) and LiCo0.5M0.5O2 (M = Mn, Fe, Ni).