Reports: DNI153543-DNI1: Concise and Logical Bottom-Up Synthetic Approach to Structurally and Electronically Designed Graphene Nanoribbons
Wesley A. Chalifoux, PhD, University of Nevada, Reno
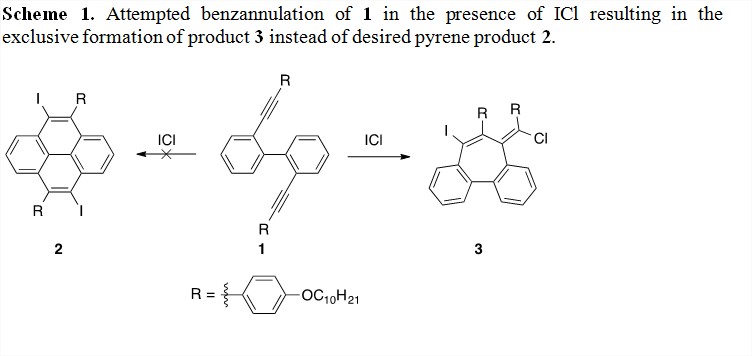
Pyrene is a small subunit of the GNRs that we intend to synthesize as a model system for the synthesis of GNRs. Our original approach was the synthesis of diyne precursor 1 that would be later cyclized using either Lewis or Brønsted acid catalysis to generate our pyrene product 2 (Scheme 1). We envisioned that the synthesis of an alkyne with an electron-rich substituent (R) would increase the reactivity of the alkyne towards acids and making the cyclization step more facile. Substrate 1 was synthesized in 6 steps from commercially available starting materials in 41% overall yield. Upon treatment of 1 with ICl, we observed unusual signals in our 1H NMR spectrum for the isolated product. Instead of undergoing a double benzannulation to give pyrene 2, as we expected, we believe a novel alkyne-alkyne dimerization occurred to provide product 3 as the sole product with clean conversion. Complete characterization of 3 is currently being done as well as further studies of this unique transformation. The preference for the alkynes to cyclize in this manner is an interesting discovery and an entry point into the synthesis of 7-membered rings seen in defective GNRs. We also screened a number of Lewis and Brønsted acids but were unable to generate 2 in good yield.
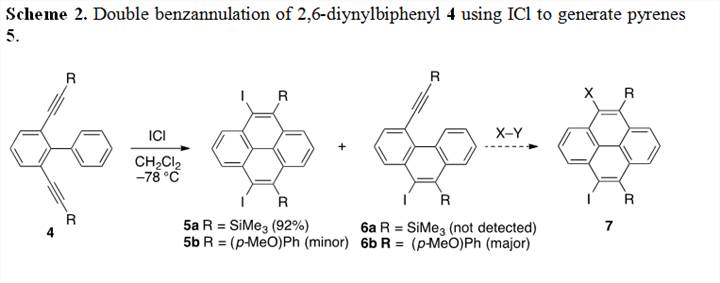
The substitution pattern of our original model compound 1 proved to be a problem if we are to utilize this alkyne benzannulation strategy for larger systems. This prompted us to turn our attention to an alternate substitution pattern shown in substrate 4 (Scheme 2). Placing both alkynes in this orientation prevents alkyne-alkyne dimerization and thus allow for our desired cyclization pathway towards pyrene products. The synthesis of 2,6-dialkynyl-1,1'-biphenyls 4 was achieved in four steps in good to excellent yields from commercially available starting materials. Treatment of 4a with two equivalents of ICl resulted in the quantitative formation of pyrene product 5a. Encouraged by this result we then synthesized and electron-rich diyne precursor 4b. The reaction of 4b with two equivalents of ICl at –78 °C for 10 minutes resulted in a mixture of double cyclized product 5b (minor product) and monocyclized product 6b (major product). This monocyclization was a fortuitous discovery as it allows us to control when the second cyclization occurs, allowing us to generate unsymmetrical pyrene products such as 7. We are currently refining the conditions warming the reaction mixture resulted in the second cyclization to occur and the clean formation of electron-rich pyrene 5b in quantitative yield.
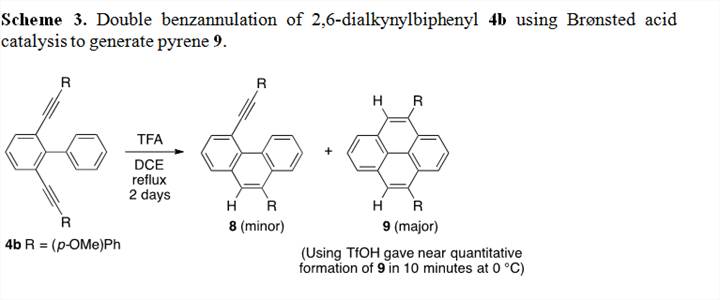
Part of our goal for the synthesis of GNRs is that the products be soluble and stable. Although the use of ICl as a benzannulation strategy seems viable and would provide a useful functional group for further chemical manipulation of the smaller oligomeric products, this would prove difficult on longer systems, especially the polymer. The incorporation of electron-rich alkynes is by design with the hope of being able to use Lewis or Brønsted acid catalysis to invoke a high-yielding polybenzannulation and provide “protonated” versions of our GNR products. The reaction of diyne 4b (R = OMe) in the presence of excess trifluoroacetic acid at reflux resulted in the rapid formation of monocyclized product 8 followed by the slow formation of pyrene 9 over 2 days. The high temperature of long reaction time needed for this transformation seems potentially problematic for larger systems. We thus attempted the same reaction with a catalytic amount of triflic acid at 0 °C, which resulted in the near quantitative formation of pyrene 9 in only 10 minutes! We are now looking at the scope of this benzannulation methodology. It should be noted that this part of the project and all of its discoveries was done entirely by undergraduate researchers. There has been a total of three undergraduate researchers that have contributed on the synthesis of pyrenes as a model system for the synthesis of GNRs. Part of this research has been presented as a poster at a recent ACS conference by one of the undergraduate researchers who has done a majority of the work on this project.
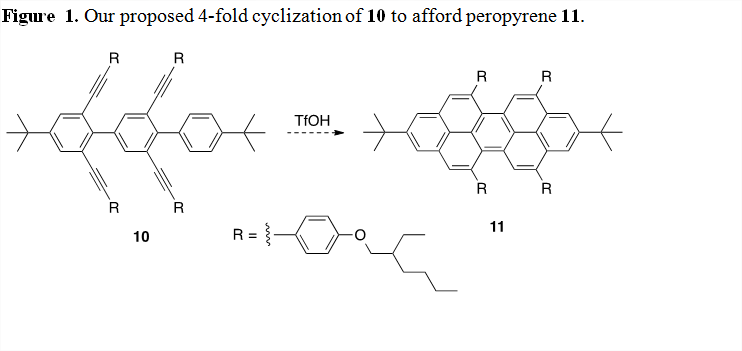
With the successful formation of our model pyrene systems using ICl, trifluoroacetic acid, and triflic acid, we are now turning our attention towards the synthesis of the next longer analogue 10 (Figure 1). This stage of the project is being undertaken by a recently hired postdoctoral scholar with the assistance of undergraduate researchers. We have made significant progress towards the synthesis of substrate 10. Once we have compound 10 in hand, we will then attempt a 4-fold benzannulation of the alkynes using triflic acid catalysis to generate 11. Following this we will develop methods to synthesize longer derivatives in this oligomeric series and the polymer.
In summary, with the assistance of the ACS-PRF fund we have collected valuable preliminary data for the rational design and synthesis of GNRs through a “bottom-up” approach. We will now use the successful methodology developed above for the synthesis of longer oligomers and polymers of GNRs.