AmericanChemicalSociety.com
Reports: B4 46543-B4: Ion Sensitive Push-Pull Pyridoannulenes
Keith C. Russell, Northern Kentucky University
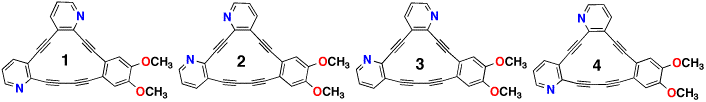
The goal of this work is for undergraduates to synthesize a series of annulenes and test their spectroscopic properties in the presence of various metal cations. The annulenes to be prepared were of two series, crown ether annulenes and "asymmetric" dimethoxy annulenes (1 - 4). Thwarted by the inability to purify the intermediates necessary to prepare the crowned annulenes, we turned our attention exclusively to annulenes 1 – 4. This report will update the status of the project since the last report.
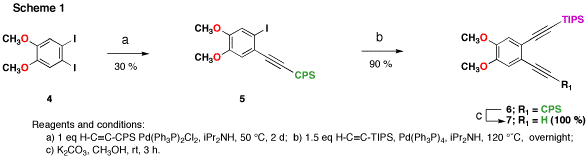
Our strategy for the preparation of these compounds of these compounds was based on our previously published synthesis. The attempted synthesis of annulene 1 serves as a model. Scheme 1 shows the synthesis mono-protected bisalkyne 8. This compound is prepared by statistical coupling of CPS-protected acetylene to diiodoanisole 4. Yields for this reaction after many attempts at optimization averages 30 %. It appears that product 5 is more ractive towards Sonogashira coupling than the starting material 4, so large quantities of decoupled material are obtained. However, even on 20 g scale, the purification by column chromatography is facile. Once isolated, compound 5 readily couples to TIPS-acetylene to afford differentially protected bis-alkyne 6. Compound 6 can be prepared on large scale and stored for long periods. The CPS protecting group can be removed in high yield prior to use.
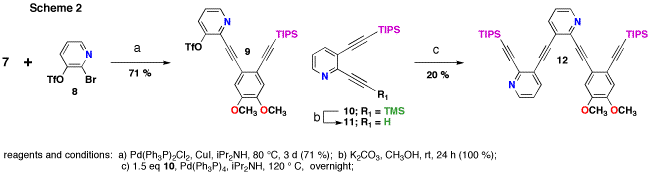
The key to the synthesis is the formation on triarene intermediate 12, the immediate precursor to annulene 1 (Scheme 2). First, partially deprotected 7 was coupled to bromotriflate 8 in good yield. Then partially deprotected bisalkynyl pyridine 11 was coupled to triflate 9. Unfortunately, the yields of this reaction were poor (ca. 20 %) and could not be scaled up. In the reaction all of the dimer 9 and pyridine 11 were consumed and several new products were formed. We believe that the product of the coupling 12, is unstable to the 120 °C reaction temperature. Attempts to do the reaction at lower temperatures for longer times either resulted in no reaction or similar amounts of decomposition. Furthermore, it was extremely difficult to completely separate the product of this reaction from the side products.
This same problem exists in the final coupling to prepare all the trimer precursors to 1 - 4. However, we have fully characterized the four trimer precursors to 1 - 4 with the exception of 13C NMR. The very small amounts of compound isolated and large MW of the products made this difficult. These problems led us to re-evaluate our synthetic approach. Our new approach is to eliminate the 120 °C coupling as the last step. This can be accomplished if compound 5 were used in the final coupling reaction. This only requires a modes deviation from the original synthsis.
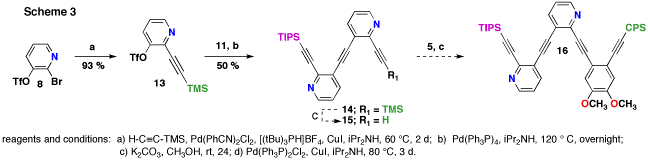
The revised (proposed) synthesis of 16 (a protected variant of 12) is shown in scheme 3. Instead of coupling the entire anisole fragment to bromotriflate 8 TMS-acetylene was coupled in high yield. The second coupling, which still requires 120 °C, uses partially deprotected bisalkynyl pyridine 11. This reaction worked considerably better than the 120 °C coupling reaction in Scheme 2. The final steps will first require deprotection, which we do not anticipate being problematic, to liberate 15. Lastly, intermediate 5 can be coupled. We estimate that the temperature for this coupling might be as low as 60 °C. That should greatly reduce the probability of decomposition.
This research has had a significant impact on all who participated in the project, as well as the PI, and other students who were not specifically involved in this area. Through presentations and the experiments themselves, we all learned a great deal with respect to handling these compounds. It was also the isolation of previously unknown dimer, mistakenly prepared, that led to the new synthetic route.
The summer had a significant impact on the PI with respect to the desired to continue working and expanding the research in this area. All of the senior students involved in the project benefited from the experience and were motivated to seek post graduate training. I am hopeful that one student, who is a rising junior, will change her major to chemistry and wishes and attend graduate school.
Copyright © American Chemical Society