AmericanChemicalSociety.com
Reports: AC7 45823-AC7: Highly Functionalized Macromolecules Based on Free Radical Copolymerization of Substituted Stilbene Monomers
S. Richard Turner, Virginia Polytechnic Institute and State University
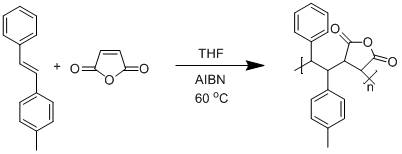
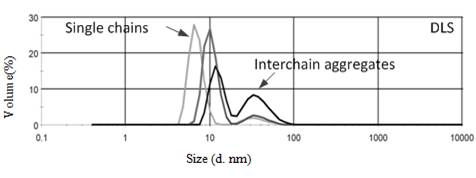
1,2-Diphenyl ethylene (stilbene) is a sterically hindered molecule that has been proven resistant to free radical, anionic, and cationic chain polymerizations. It however readily polymerizes free radically, in a strictly alternating fashion, with maleic anhydride and N-substituted maleimides. The research in this project is focused on preparing new substituted stilbene monomers and copolymerizing them with maleic anhydride and N-substituted maleimide comonomers. The first thrust was to bring to a conclusion a study on the influence of methyl substituents on the phenyl group of the stilbene units. This chemistry is exemplified in Scheme 1. We discovered bimodal peaks both in Dynamic Light Scattering (DLS) curves shown in Figure 1 and in Size Exclusion Chromatography (SEC) curves with the copolymerization of 4-methylstilbene and maleic anhydride. We also studied the volume change of the peaks with solution concentrations by DLS. It was found that the volume of the peak for the large sized molecules deceased as the solution concentration decreased which is consistent with interchain aggregation. We also observed the interchain aggregates decrease in size when subjected to ultrasonic treatment and then recovered in size.
Scheme 1
Figure 1
These polymer aggregates likely originate from self association of specific steric sequences along the sterically crowded backbones in the stilbene-maleic anhydride copolymers and these unique structures could be involved in the self-association. Such effects have been observed and reported in a recent publication for styrene-maleic anhydride and styrene-N-substituted maleimide alternating copolymers where novel nanostructures are formed by self-assembly of various sequences along the backbone (1). Our solid state NMR work showed the maleic anhydride units are enchained in a predominately cis configuration (2). This type of self association potentially opens up a new area for future research with these copolymeric systems as a route to form highly functional nanoparticles.
These details of this thrust of this study are contained in a recent publication (3).
A second thrust of this work was to study the effect of an external stimulus, sodium chloride, on the polyelectrolyte complexes of these steric crowded rigidized stilbene polyelectrolytes and compare to known flexible system. This work was prompted by our initial work in the project that indicated that the sterically crowded stilbene monomers, when incorporated into the backbone, led to an increase in chain stiffness which resulted in "like-charge attraction" for zwitterionic block copolymers (4, 5).
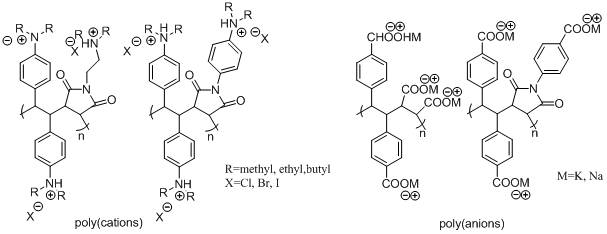
For this study we prepared positively charged polyelectrolytes based on ammonium salts and negatively charged polyelectrolytes based on carboxylate salts shown in Figure 2.
Figure 2
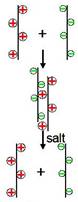
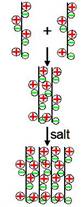
Preliminary results indicated that these anion and cation polyelectrolyte complexes fall apart with the addition of salt while rigid zwitterions do not, as shown in Figure 3.
Figure 3
References:
1. Lazzara, T.D.; van de Ven, T.G.M.; Whitehead, M.A. "Nanotube Self-Assembly of a Styrene and Maleimide Alternating Copolymer" Macromolecules 41, 6747 (2008).
2. Turner, S. R.; Mao.M.; Kim, C.; Wi, S. "Chain Structure of Substituted Stilbene-Maleic Anhydride Alternating Copolymer Probed by Solid State NMR" Macromolecules 41, 387 (2008).
3. Turner, S. R.; Li, Y. "Free Radical Copolymerization of Methyl Substituted Stilbenes with Maleic Anhydride" Eur. Polym. J. 46, 821 (2010).
4. Turner, S. R.; Mao, M. "Synthesis and characterization of highly functionalized polymers based on N,N,N',N'-tetraalkyl-4,4'-diaminostilbene and maleic anhydride" Polymer 47, 8101 (2006).
5. Turner, S. R.; Mao, M. "Aggregation of Rod-Coil Block Copolymers Containing Rigid Polyampholyte Blocks in Aqueous Solution" JACS 129, 3832 (2007).
Copyright © American Chemical Society