AmericanChemicalSociety.com
Reports: DNI10 49903-DNI10: Processing glassy polymeric membranes with enhanced thermal and kinetic stability
Rodney D. Priestley, PhD, Princeton University
Since the initial discovery that polystyrene (PS) films supported on silica exhibited a reduction in Tg with decreasing thickness [1], whereas poly(methyl methacrylate) (PMMA) films exhibited a thickness dependent Tg that was impacted by the type of supporting substrate [2], the Tg-confinement effect has been attributed to the presence of interfacial effects perturbing glass transition dynamics. Nanoscale confinement and interfacial effects also impact, physical aging, i.e., glassy-state structural relaxation [3,4]. We have shown that the dispersion of nanofiller into a polymer matrix, in which there exists an attraction between the nanofiller and polymer, can lead to suppressed or nearly no physical aging of polymer nanocomposite films [5,6].
A factor affecting the stability or long-term throughput of glassy polymeric membranes is physical aging [7,8]. Physical aging leads to a decrease in free volume [9]. For glassy membranes the permeability can decrease greater than forty percent over a modest lifetime due to physical aging. In this proposal, we investigate the stability of thin glassy polymeric membranes by Matrix Assisted Pulsed Laser Evaporation (MAPLE) and explore their use as stable ultra-barrier membranes.
Figure 1:
A) Heat capacity of ordinary and MAPLE-Deposited PMMA. B) Reversing heat capacity of ordinary
and MAPLE-Deposited PMMA as a function of time at Tg.
We will continue to investigate
the impact of substrate temperature and deposition rate on the glass
transition, stability and energy of MAPLE-deposited PMMA films and other
polymers. We are also interested
in understanding the molecular structure of MAPLE-deposited PMMA films, and
therefore plan to use x-ray scattering to probe the structure. Lastly, we will undertake barrier
studies on MAPLE-deposited films. Career and educational impact. Funding of this proposal has allowed
our group to initially explore the impact of nanoscale
confinement and interfaces on Tg and
physical aging of confined polymer, and to investigate the stability of glassy
polymeric films deposited by MAPLE.
Our work is the first to show that MAPLE-deposited glassy polymeric
films exhibit a significant enhancement in thermal and kinetic stability. A postdoctoral fellow and a graduate
student have carried out this work.
Funding of this proposal has allowed them to develop a fundamental
understanding of confinement effects on the properties of polymers and develop
the ability to process films by MAPLE. References:
temperature in thin polymer films. Faraday Discuss. 1994,
98, 219-230 3. Huang, Y.; Paul, D.R. Effect of
Temperature on Physical Aging of Thin Glassy Polymer Films. Macromolecules
2005, 38, 10148-10154 4. Huang, Y.; Paul,
D.R. Physical Aging of Thin Glassy Polymer Films Monitored by Optical
Properties. Macromolecules 2006, 39, 1554-1559 5. Priestley, R.D.; Rittigstein, P.; Broadbelt, L.J.;
Fukao, K.; Torkelson,
J.M. Evidence for the
Molecular-scale Origin of the Suppression of Physical Aging in Confined
Polymer: Fluorescence and Dielectric
Spectroscopy Studies of Polymer-Silica Nanocomposites.
J. Phys.: Condens. Matter
2007, 19, 205120-1-205120-12 6. Rittigstein,
P.; Priestley, R.D.; Broadbelt, L.J.; Torkelson, J.M. Model Polymer Nanocomposites
Provide an Understanding of Confinement Effects in Real Nanocomposites.
Nature Materials 2007, 6, 278-282 7. Pfromm, P.H; Koros, W.J.
Accelerated physical aging of thin glassy polymer films: evidence from
gas transport measurements. Polymer 1995, 36, 2379- 2387 8. Huang, Y.; Paul, D.R. Physical Aging in
Thin Glassy Polymer Films Monitored by Gas Permeability. Polymer 2004, 45,
8377-8393 9. Struik, L.C.E.
Physical Aging of Amorphous Polymers and Other Materials. 1978, Elesvier, Amsterdam
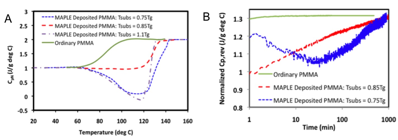 We have
completed an investigation of the glass transition and stability of supported
PMMA films deposited by MAPLE. A
main result of this work, as illustrated in Figure 1A, is that, via MAPLE, we
have been able to prepare films of PMMA that exhibit a remarkable enhancement
in thermal stability (i.e., ~ 50 K increase in the glass transition temperature
(Tg)) in comparison to ordinary PMMA. We have determined the importance of
substrate temperature in the formation of stable, high Tg
PMMA films when deposited at a constant deposition rate of ~ 5 nm/sec; films
not deposited at ~ 0.85Tg exhibited large endothermic peaks during
heating, an indication of glassy-state structural relaxation, i.e.,
instability. Furthermore, we have
observed a dependence of deposition rate on the enhancement of thermal
stability, i.e., a slower rate of deposition leads to a higher Tg. The results demonstrate, for the first time,
the formation of a thermally stable polymer film by MAPLE. A second significant finding of this
work, as illustrated in Figure 1B, it that when isothermally annealed at Tg (Tg of
the corresponding film), the time required for the glass to liquid transformation
is ~ 2-orders-of-magnitude greater for MAPLE-deposited PMMA than ordinary
PMMA. In addition, MAPLE-deposited
films that exhibit large endothermic peaks also exhibit minima in the Cp,rev vs. time curves.
We have
completed an investigation of the glass transition and stability of supported
PMMA films deposited by MAPLE. A
main result of this work, as illustrated in Figure 1A, is that, via MAPLE, we
have been able to prepare films of PMMA that exhibit a remarkable enhancement
in thermal stability (i.e., ~ 50 K increase in the glass transition temperature
(Tg)) in comparison to ordinary PMMA. We have determined the importance of
substrate temperature in the formation of stable, high Tg
PMMA films when deposited at a constant deposition rate of ~ 5 nm/sec; films
not deposited at ~ 0.85Tg exhibited large endothermic peaks during
heating, an indication of glassy-state structural relaxation, i.e.,
instability. Furthermore, we have
observed a dependence of deposition rate on the enhancement of thermal
stability, i.e., a slower rate of deposition leads to a higher Tg. The results demonstrate, for the first time,
the formation of a thermally stable polymer film by MAPLE. A second significant finding of this
work, as illustrated in Figure 1B, it that when isothermally annealed at Tg (Tg of
the corresponding film), the time required for the glass to liquid transformation
is ~ 2-orders-of-magnitude greater for MAPLE-deposited PMMA than ordinary
PMMA. In addition, MAPLE-deposited
films that exhibit large endothermic peaks also exhibit minima in the Cp,rev vs. time curves.
Copyright © American Chemical Society

