AmericanChemicalSociety.com
Reports: UNI5 50108-UNI5: Probing the Dynamics of Ion Transport by Scanning Electrochemical Microscopy: Towards the Development of Enhanced Fuel Cell Membranes
Marisa C. Buzzeo, DPhil, Barnard College
We seek to understand the factors that most strongly govern the processes of ion transport and charge separation across synthetic membranes using scanning electrochemical microscopy (SECM). By systematically varying both membrane composition and environmental conditions, the permeability, conductivity, and operating range of candidate materials can be evaluated. We believe the information acquired through such experiments will ultimately allow for the development of more efficient fuel cells as a result of improved membrane design.
Our efforts to date have largely focused on outfitting our new research laboratory (which became available in November 2009 following major renovation) and training undergraduate research students. In addition to standard lab equipment, our research space now houses two conventional potentiostats and, perhaps most importantly, one SECM (with its own bipotentiostat). All of these instruments have been used towards preliminary membrane studies. Three undergraduate students (Bianca Lahiji, BC '10, Nanette Jarenwattananon, BC '11, and Michelle Sykes, BC '12) have been trained on the equipment, and a fourth student (Camille Gandara, BC '12) has most recently joined the SECM/membrane project. A significant portion of the past year has been dedicated to introducing these students to the lab environment, the fundamentals of electrochemistry, and the operating principles of SECM. As a result, our most early studies focused on calibrating and characterizing well-behaved redox systems, such as ferrocene and potassium ferricyanide. Parallel studies on macro- and microelectrodes allowed the students to gain an appreciation of the influence of surface geometry on the observed voltammetry. Within the past few months, our SECM-based investigative efforts have begun and, encouragingly, have already yielded positive results.
SECM
is well-suited for studying transport across membranes, as has been demonstrated
previously. The intrinsic two-electrode design allows for electrochemical
interrogation on both sides of a suspended surface, as well as the
establishment of a potential gradient across the membrane. Both of these
features have significant implications for studies of ion transport. We aim to
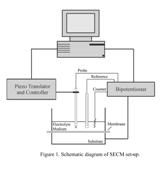
devise a cell arrangement analogous to that shown in Figure 1, whereby the
synthetic membrane under study would be mounted between the substrate and tip
electrodes in the presence of a given solvent. Initial studies, however, have
utilized naturally self-assembling phospholipid bilayers as a model system.
Importantly, solutions of these lipids can be directly deposited onto the SECM
substrate electrode and do not require a modified cell set-up. The ease of deposition
and surface modification translates to increased repeatability and data
collection, which is of particular importance in the early stages of these
experiments as we refine the practical details.
In
order to establish a membrane surface that can in fact be electrochemically
analyzed from either side, it is useful to employ a passivating agent that
serves both to suspend the membrane above the substrate electrode as well as
block diffusion of redox-active species to the electrode surface. Effective
passivating agents are also known to provide This
grant has been instrumental to the early development of our research program
and we are grateful to the Petroleum Research Fund for this support. In addition to funding various aspects of the
experiments described above, the award financed travel to and attendance at the
National ACS Meeting in Boston this past August, where our results to date were
presented. We believe our preliminary findings show great promise for the
application of scanning electrochemical microscopy to the proposed membrane
studies and look forward to pursuing these experiments further.
 an added level
of structural support to self-assembled monolayers, and thus, in this case, to
the overall construct of the membrane. A number of different alkanethiol chains
were tested at various concentrations and incubation times. It was found that
octadecanethiol (ODT) or mixed monolayers of ODT and mercaptohexanol
demonstrated the most reliable and reproducible passivation against
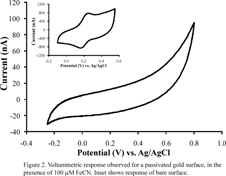
solution-borne potassium ferricyanide (FeCN). Figure 2 shows a typical voltammetric
response at a gold electrode that has been incubated with 200 mM ODT for one hour and then
exposed to 100 mM ferricyanide.
It is evident from the absence of faradaic features in the potential window
shown that the FeCN molecules are no longer free to diffuse to the surface.
(The inset shows the voltammetric response of FeCN at bare gold for
comparison). Commercially-available
phospholipids, specifically L-a-phospatidylcholine
and 1,2-dioleoyl-sn-glycero-3-phospho-L-serine, were then employed as self-assembling
membrane materials. Following passivation of the surface, extruded solutions of
lipid vesicles were deposited onto the modified electrode and analogous
voltammetric measurements were taken. It was consistently observed that the
degree of passivation against FeCN was either retained or even improved
following addition of lipids to the surface.
an added level
of structural support to self-assembled monolayers, and thus, in this case, to
the overall construct of the membrane. A number of different alkanethiol chains
were tested at various concentrations and incubation times. It was found that
octadecanethiol (ODT) or mixed monolayers of ODT and mercaptohexanol
demonstrated the most reliable and reproducible passivation against
solution-borne potassium ferricyanide (FeCN). Figure 2 shows a typical voltammetric
response at a gold electrode that has been incubated with 200 mM ODT for one hour and then
exposed to 100 mM ferricyanide.
It is evident from the absence of faradaic features in the potential window
shown that the FeCN molecules are no longer free to diffuse to the surface.
(The inset shows the voltammetric response of FeCN at bare gold for
comparison). Commercially-available
phospholipids, specifically L-a-phospatidylcholine
and 1,2-dioleoyl-sn-glycero-3-phospho-L-serine, were then employed as self-assembling
membrane materials. Following passivation of the surface, extruded solutions of
lipid vesicles were deposited onto the modified electrode and analogous
voltammetric measurements were taken. It was consistently observed that the
degree of passivation against FeCN was either retained or even improved
following addition of lipids to the surface. 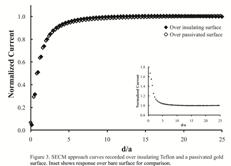 Most recently,
we have begun to conduct such studies using SECM. Similar voltammetric
responses were observed when the substrate gold electrode was modified with
octadecanethiol. In order to provide further evidence for the presence of this
passivating layer, we recorded approach curves, whereby the current response at
the probe electrode is measured as it is lowered closer and closer to the
substrate surface, in the presence of FeCN. When such experiments are recorded
over conductive substrates (e.g. bare gold), the current readings will display
positive feedback that arises from the ability of reduced FeCN to be
re-oxidized at the substrate electrode. Over an insulating surface (e.g. PTFE),
the current will exhibit negative feedback, as the FeCN molecules are blocked
from the surface. Approach curves were measured over the bare gold substrate
electrode, the Teflon casing that surrounds the substrate, and the gold surface
modified with ODT. The current response for the latter two was nearly
identical, indicating the surface had been effectively passivated against FeCN
(see Figure 3). Current studies are focused on using SECM to evaluate more
closely the formation of these bilayers as well as monitor ion transport across
them. We are also designing an
electrochemical cell that would accommodate synthetic membranes so that we can
begin surveying commercially-available materials. Future work will employ electrochemical
impedance spectroscopy, quartz crystal microbalance, and atomic force
microscopy for detailed characterization studies of these surfaces.
Most recently,
we have begun to conduct such studies using SECM. Similar voltammetric
responses were observed when the substrate gold electrode was modified with
octadecanethiol. In order to provide further evidence for the presence of this
passivating layer, we recorded approach curves, whereby the current response at
the probe electrode is measured as it is lowered closer and closer to the
substrate surface, in the presence of FeCN. When such experiments are recorded
over conductive substrates (e.g. bare gold), the current readings will display
positive feedback that arises from the ability of reduced FeCN to be
re-oxidized at the substrate electrode. Over an insulating surface (e.g. PTFE),
the current will exhibit negative feedback, as the FeCN molecules are blocked
from the surface. Approach curves were measured over the bare gold substrate
electrode, the Teflon casing that surrounds the substrate, and the gold surface
modified with ODT. The current response for the latter two was nearly
identical, indicating the surface had been effectively passivated against FeCN
(see Figure 3). Current studies are focused on using SECM to evaluate more
closely the formation of these bilayers as well as monitor ion transport across
them. We are also designing an
electrochemical cell that would accommodate synthetic membranes so that we can
begin surveying commercially-available materials. Future work will employ electrochemical
impedance spectroscopy, quartz crystal microbalance, and atomic force
microscopy for detailed characterization studies of these surfaces.
Copyright © American Chemical Society

