AmericanChemicalSociety.com
Reports: AC9 47976-AC9: Liquid Crystalline Membranes for Olefin/Paraffin Separations
Stephen M. Martin, Virginia Polytechnic Institute and State University and Eva Marand, Virginia Polytechnic Institute and State University
We are developing liquid crystal (LC) and liquid crystal polymer (LCP) based membranes which have selectivity targeted for olefin/paraffin separations using structural regularity and specific molecular interactions. The primary challenges being addressed include (1) the controlled fabrication of stable, defect-free membranes and characterization of the resulting liquid crystalline order, and (2) the characterization of transport properties and selectivities for an olefin/paraffin gas pair, in this case propane/propylene, as a function of liquid crystalline order and molecular structure. The goal is to enhance solubility and diffusion of the targeted molecules by enhancing molecular interactions and the ability of the gas component to fit into the liquid crystalline structure. <>2 Research Impact <>2.1 Supported Liquid Crystal Membranes:
Fabrication of Supported LC Membranes: Membranes consisting of a porous support impregnated with an LC material have proven useful in the evaluation of LC transport properties in solution-based separations. LC embedded membranes are prepared by impregnating the porous cellulose nitrate membrane with an LC material, such as 4-cyano-4'-octylbiphenyl (8CB) via vacuum filtration
Transport in Supported LC Membranes: A constant volume/variable pressure apparatus was used for transport property measurement. Under testing, the liquid crystals separated from the support. This was due to the increase of pressure (1.5–2.0 atm) on the feed side, which overcomes the capillary forces keeping the LC material in the porous support. For this reason, we concentrated on developing more physically robust membrane systems, such as polymer dispersed LCs (PDLCs.) <>2.2 Polymer-dispersed Liquid Crystal Membranes:
Figure 1: SEM images of the cross-section of a 55wt% 8CB/polysulfone.
Polymer-dispersed Liquid Crystal
(PDLC) Membranes: PDLC membranes
are phase-separated structures consisting of pockets of the LC material
dispersed in a polymer matrix (Figure 1.) As a result, they are more stable to applied
pressure than the supported LC membranes. A polysulfone/LC mixture is dissolved
in chloroform and cast on a glass substrate. The size and number of domains in
the membrane can be controlled via a temperature annealing step. At high
concentrations, the LC forms of separate macroscopic nematic and polymer
domains which lead to decreased membrane stability.
Transport in Polymer-dispersed
Liquid Crystal (PDLC) Membranes: We measured the permeation of propane
and propylene in pure polysulfone, and 8CB/polysulfone PDLC membranes (Table 1.) The data indicate some improvement in selectivity;
however, these membranes exhibit poor physical stability.
Table 1: Permeability and
selectivity for PDLC membranes produced using 8CB and polysulfone.
Permeability (barrers) 37 oC 50 oC Pure Polysulfone 6.4E6* 3.3E5* 34 wt% 8CB in PS 1.8E3 1.9E5 55 wt% 8CB in PS 2.0E3 4.6E3
Selectivity 37 oC 50 oC Pure Polysulfone 1.2 1.2 35 wt% 8CB in PS 2.1 1.1 55 wt% 8CB in PS 4.4 4.3
<>2.3 Side-chain
Mesogenic Polymer Membranes:
Materials and Fabrication: We
obtained a butadiene based side-chain polymer from the Institute of Macromolecular
Physics, Academy of Sciences of the Czech Republic. The polymer contained a cyanobiphenyl mesogen
and a six carbon spacer (Figure 3). The telechelic poly(butadiene)diol backbone
had an average molecular weight of approximately 2800 Da with 88 mol% mesogen
functionalization. With such a high degree of functionalization, the polymer
did not exhibit a liquid crystalline phase. We used an impregnation method to
fabricate the membranes in a porous support.
DSC indicated all transition temperatures were identical to the pure
polymer. SEM images confirm complete impregnation of the material in the
support.
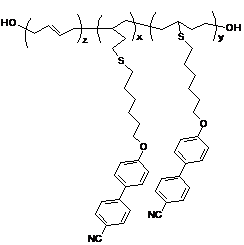
Figure
3: Polymer structure of the butadiene backbone and cyanobiphenyl based
side-chains.
Transport in
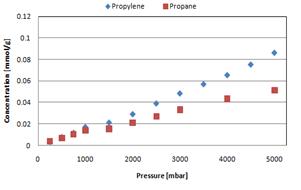
Side-chain Polymer Membranes: Figure 5: Single gas propylene and propane sorption (left) 35 °C
and (right) 50 °C.
Sorption isotherms of propane and
propylene indicated a sorption selectivity of ~2 that remained constant when
varying both pressure and temperature. Gas
sorption increased with temperature. Permeability measurements for membrane
transport of both single and mixed gases were performed using a constant
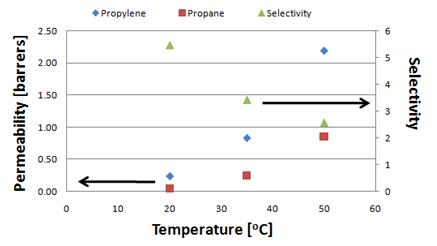
volume-variable pressure and constant pressure-variable volume apparatus. Permeation measurements at room temperature,
the lowest temperature studied, exhibited the greatest permselectivity (Figure
6.) Permeation of both propylene and
propane increased with temperature.
Figure 6: Single gas propylene and propane permeability and
selectivities.
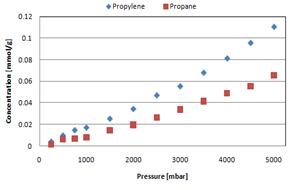
Mixed gas permeability
measurements indicate that the permeabilities of both propylene and propane
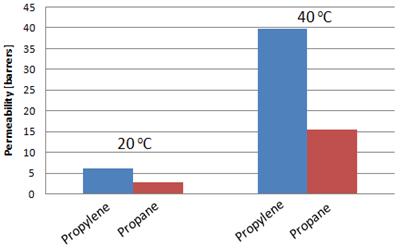
increase with temperature, with no decrease in selectivity (Figure 7.) Overall permeability increased and
selectivity decreased slightly compared to single gas measurements. The
permeability and selectivity in the side-chain crystalline polymer are
promising, and we intend to study related polymers with lower fractions of
mesogen functionality in which liquid crystalline phases are observed. In the interim, we pursued the possibility of
inducing LC phases by blending the crystalline polymer with structurally
homologous small molecule liquid crystals.
Figure
7: Mixed gas 50/50 propylene/propane permeability results in BCP membranes
at 20 °C
and 40 °C. 3
Blended Polymer/Liquid Crystal Membranes:
Due to the lack of an LC phase
due to the high degree of functionalization, we have begun blending a small molecule
thermotropic liquid crystal with the polymer. The addition of a small organic
molecule should serve to plasticize the crystalline polymer, leading to an
increase in mobility and permeability and a possible LC phase. DSC measurements
indicate decreased melting temperature with added 8CB. Permeation of propylene in a 10wt% 8CB
membrane increased by a factor of 6 with only a slight reduction of selectivity
compared to the pure polymer membrane. 4
Career Impact:
The work has provided support for
one graduate student early in his PhD career and one graduate student at the
end of his PhD career. They developed expertise in membrane fabrication and
characterization (microscopy, SEM), transport property measurement, and has
gained experience in the design, fabrication, and modification of laboratory
instrumentation. In addition, the graduate student has been mentoring an
undergraduate researcher, providing valuable lab experience for the
undergraduate student and leadership experience for the graduate student. The
project has had a significant impact on the career development of Dr. Martin,
as it has allowed him to develop expertise and instrumentation in membrane
characterization and to begin exploring the area of mixed matrix (PDLC,) liquid
crystal polymer (LCP,) and blended LC/polymer membranes.

Copyright © American Chemical Society