AmericanChemicalSociety.com
Reports: B1 47986-B1: Preparation of Mannich Bases and Their Corresponding Silyl Enol Ethers from 2-Acylaziridines
Aaron M. Hartel, Winthrop University
Mannich bases (b-aminoketones) and their derivatives are important synthetic intermediates, particularly in the preparation of biologically active molecules. The traditional method for their preparation is the Mannich reaction, however, this method has many drawbacks such as long reaction times, poor regioselectivity, no enantioselectivity, and competition from unwanted side reactions.
We are developing new methods for the preparation of Mannich bases from 2-acylaziridines through intermediary proximal b-amino silyl enol ethers. These functionalized silyl enol ethers should react with a variety of electrophiles to provide Mannich bases of varied structural complexity.
During the first two years of this project, we have focused on developing two separate but related methods for the stereo- and regioselective preparation of the Mannich bases and proximal b-amino silyl enol ethers, respectively. The central reaction common to both methods is the reaction of a 2-acylaziridine with a silyllithium reagent, which triggers a Brook rearrangement with concomitant opening of the adjacent aziridine ring.
For the direct formation of simple Mannich bases, an excess of silyllithium reagent is used and conditions have been adjusted to promote the in situ cleavage of the intermediate silyl enol ether, which provides the Mannich base on work up.
Optimization studies were
performed using N-acetyl-2-benzoyl-3-methylaziridine and 2-acetyl-3,3-dimethyl-N-tosylaziridine as representative aryl
and alkyl ketones substrates, respectively. As anticipated, it was found that solvents
such as THF that strongly bind to lithium ions gave the highest yield of Mannich base. Use of
nonpolar solvents such as ether or toluene retarded
the necessary desilylation, resulting in lower yields
of the desired Mannich base. The most commonly encountered silyllithium reagent, dimethylphenylsilyllithium,
was found to efficiently effect the reaction of the aryl ketone
substrate, N-acetyl-2-benzoyl-3-methylaziridine. However, reaction of
the alkyl ketone substrate, 2-acetyl-3,3-dimethyl-N-tosylaziridine, required the use of methyldiphenylsilyllithium to achieve an acceptable yield
of Mannich base.
The lack of an electron-withdrawing group at the reaction site of the
alkyl ketone necessitated additional electron
withdrawal from the incorporation of a second phenyl substituent on the silyl group.
Overall, the combination of excess methyldiphenylsilyllithium
in THF at -40 °C proved to be a convenient system that gave the desired Mannich base in good yield from either the alkyl or aryl
substituted substrate. Several
differentially substituted 2-acylaziridine substrates have been prepared and
reacted using these optimized conditions to determine the scope of the
method. Both aryl and alkyl 2-acylaziridines
reacted to give the corresponding Mannich bases in
good to excellent yields. The method is
compatible with amide, carbamate and sulfonamide
protecting groups on the aziridine nitrogen, but
ineffective for use with an unprotected nitrogen.
Substantial progress has also
been made on the second method of the project, the preparation of proximal b-amino
silyl enol ethers. Initial optimization studies have been
performed using N-acetyl-2-benzoyl-3-methylaziridine as an investigative
substrate. Use of nonpolar solvents such as ether or
toluene retarded the desilylation providing mixtures
of Mannich base and the desired b-amino
silyl enol ether. Variations on the silyllithium
reagent have also been investigated.
Reaction of N-acetyl-2-benzoyl-3-methylaziridine with dimethylphenylsilyllithium provided the desired b-amino
silyl enol ether in low
yield. Use of methyldiphenylsilyllithium
resulted in a substantially higher yield of the silyl
enol ether, but was accompanied by a substantial
amount of undesired Mannich base. Increasing the steric
bulk of the silyl group was investigated as a
potential means to retard the undesired desilylation
of the silyl enol ether.
Use of t-butyldiphenylsilyllithium was found
to completely suppress the cleavage of the silyl enol ether.
Future work on the direct
preparation of Mannich bases will include the
reaction of additional substrates to further demonstrate the generality of the
method. For the preparation of b-amino
silyl enol ethers,
additional optimization studies will be performed. A variety of N-protecting groups will be
investigated to determine the group's influence on the suppression of the desilylation. We
will also investigate whether the use of t-butyldiphenylsilyllithium
can prevent cleavage of the sily enol
ether even in solvents such as THF which typically promote desilylation
but which also promote the Brook rearrangement.
This may be crucial for the successful reaction of alkyl
2-acylaziridines, which are expected to undergo Brook rearrangement much more
slowly than the aryl 2-acylaziridines investigated so far.
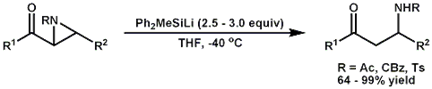
Copyright © American Chemical Society


