AmericanChemicalSociety.com
Reports: B3 48348-B3: Fundamental Studies of Metalloporphyrin Catalyzed Oxidation of Dibenzothiophenes
Alan Gengenbach, PhD, University of Wisconsin (Eau Claire)
Dibenzothiophenes (DBTs) are sulfur-containing aromatic compounds found in diesel and other fuel stocks. The traditional hydrodesulfurization (HDS) treatment process does not remove these compounds efficiently. Therefore, HDS is not suitable for producing fuels that meet the more stringent standards for Ultra-Low Sulfur Diesel. Oxidative desulfurization (ODS) is currently receiving significant attention as an alternative to HDS. ODS is a two-step process where chemical oxidation of the sulfur compounds is followed by extraction of the oxidized products from the fuel. The reaction of the DBTs with oxidants typically requires the use of a catalyst. The goal of this project is a detailed understanding of the metalloporphyrin catalyzed oxidation of DBTs.
During the 2008-2009 funding period, we found that
reactions performed in a CH2Cl2/CH3CN/methanol
mixture with 50 mM substrate, 5 equivalents of hydrogen peroxide and 1%
catalyst gave conversions of >99%, 98%, 97% and 46% for BT, DBT, 4-MDBT and
4,6-DMDBT, respectively. The sulfoxide and sulfone derivatives of DBT were
identified as the products of the reaction by GC-MS. For all substrates, both
the sulfoxide and sulfone products were observed by GC-MS, and minimal
conversion was observed in the absence of the catalyst. These results
demonstrated that Fe(PFPP)Cl catalyzes for the oxidation of DBTs. The reaction conditions were modified to investigate how
well the reaction proceeds when octane replaced CH2Cl2/CH3CN
as the main solvent. High conversions were observed using only a minor excess
of oxidant when the reactions were performed with substrate concentrations of
50 mM. Since concentrations around 200 ppm for DBT, BT, 4-MDBT and 4,6-DMDBT are
more typical for real fuels, we modeled fuels using an octane solution
containing all four substrates at a concentration of 200 ppm. Preliminary
reactions with the model fuel showed that larger excesses of oxidant are
required to achieve good conversions in reasonable time periods. During 2009-2010, we continued the model fuel studies. Oxidation
of the sulfur compounds was achieved using 10 equivalents (relative to total
sulfur) of hydrogen peroxide in the presence of 1% Fe(TPFPP)Cl. GC-MS of the
oxidized model fuel revealed a mixture of sulfoxide and sulfone products. The observed conversions of BT, DBT, 4-MDBT
and DMDBT were 70%, 85%, 65% and 24%, respectively. The model fuel was
subjected to a simple oxidation-extraction scheme. For comparison purposes,
samples of reacted and unreacted model fuel were extracted with methanol.
Extraction of the unoxidized model fuel reduced the BT, DBT, 4-MDBT and
4,6-DMDBT concentrations in the model fuel by 68%, 55%, 45% and 28%, which
corresponds to a reduction of the total S content in the model fuel of 50%.
Oxidation-extraction of the model fuel dropped the BT, DBT, 4-MDBT and
4,6-DMDBT concentrations in the model fuel by 98%, 95%, 94% and 77%,
respectively. Therefore, oxidation-reduction treatment decreased the total sulfur
content of the model fuel by approximately 91%.
These model fuel experiments showed that complete conversion of the DBT
derivatives to either the sulfoxide or sulfone is sufficient to facilitate
their extraction from a hydrocarbon phase. Most published ODS schemes involve oxidation of DBT and
related compounds to the corresponding sulfones prior to extraction. The
reported reactions are typically optimized to maximize the yield of sulfones,
presumably because the sulfones are more polar than the corresponding
sulfoxides. As a result, the literature suggests that high conversion to the
sulfones is necessary for successful extraction. To provide further support for
our contradictory observations, experiments to determine the extraction
efficiencies of both the sulfoxides and sulfones were initiated. Since only DBT
sulfone is commercially available, these experiments required synthesis of the
desired compounds. Sufficient quantities of DBT sulfoxide, 4-MDBT sulfoxide,
and 4,6-DMDBT sulfoxide were synthesized from the sulfides using a slight
excess of hydrogen peroxide and Fe(TDCPP)Cl as the catalyst. The products were
isolated and purified using chromatography on silica gel. We observed yields
and selectivities for the sulfoxides that matched or exceeded those reported
previously. 4-MDBT sulfone and 4,6-DMDBT sulfone could not be obtained simply
by increasing the amount of hydrogen peroxide used in the reaction. Therefore, the sulfones were obtained by
reacting the sulfides with excess MCPBA. 20 ppm solutions of DBT, DBT sulfoxide and DBT sulfone
were extracted with methanol. The octane layers were analyzed by HPLC before
and after treatment with methanol. Based
on qualitative comparisons of the peak areas, both the DBT sulfoxide and DBT sulfone
were more efficiently removed than DBT.
Furthermore, while some DBT sulfone remained in the octane layer, DBT
sulfoxide was not detected in the octane layer following methanol extraction.
Therefore, although we have not precisely determined the partition
coefficients, these preliminary results suggest that the sulfoxide is extracted
at least as efficiently as the sulfone. Since complete conversion of sulfide to
sulfone requires at least 100% more oxidant than conversion to the sulfoxide, ODS
treatment processes would be more economical if the sulfoxides can be
efficiently extracted from fuels. To more rigorously support our hypothesis,
octanol-water partition coefficients for these compounds will be measured using
standard protocols. Funding of this project is extremely important for the
PIs overall research program and the undergraduates working on the project. To
date, four UWEC students have worked on this project and three students are
currently being supported. Another
undergraduate will start work on the project in the Spring of 2011. Dan Swedien
and Erin Stuckert presented their work at the National ACS meeting in San
Francisco using travel funds provided by this grant. These students will
graduate at the end of this academic year and both will continue their
education. Dan is applying to Medical and MD/Ph. D programs and Erin is
applying to chemistry Ph. D. programs. This
project provided a suitable research experience for Charles Thurber (UW-Fox
Valley) for the 2010 summer with additional funding provided by the PIs NSF-REU
grant. Mr. Thurber is applying for admission to UWEC as a transfer student.
Once admitted, it is expected that he will continue to work on this project.
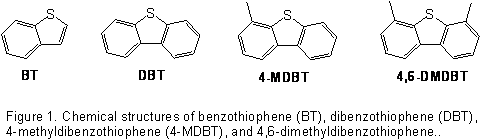
Copyright © American Chemical Society

