AmericanChemicalSociety.com
Reports: AC10 47379-AC10: First-Principles Studies of Water Splitting at Semiconductor and Oxide Surfaces
Chris G. Van de Walle, University of California (Santa Barbara)
The need to decrease the dependence on energy from carbon-based sources is widely recognized and renewable-energy alternatives are actively being pursued. The most promising technologies are wind turbines and solar cells. However, these sources deliver electricity literally only as the wind blows or the sun shines. Thus, efficient energy storage will be essential in a society where a significant fraction of energy is delivered by renewable sources. One possibility is to convert electrical into chemical energy using electrolysis, splitting water into hydrogen and oxygen.
In the present project we go one step further and study direct hydrogen production by a photoelectrochemical cell, which combines a solar cell and an electrolysis cell into one device and produces hydrogen directly without the loss inherent in the traditional two-step process. One of the electrodes consists of a semiconductor that absorbs sunlight and produces electrons and holes, which split water into hydrogen and oxygen.
We have earlier demonstrated that density functional theory (DFT) calculations based on advanced functionals (such as the HSE06 hybrid functional) produce band gaps in close agreement with experiment, which is essential for accurately calculating alloy band gaps and band alignments on an absolute energy scale. Our results thus indicate that DFT with HSE06 can give valuable insight into key properties in photoelectrochemistry.
Here we describe our recent results on the methodology of calculating band alignments and our study of onset potential for water oxidation on oxide and nitride surfaces.
Methodology for calculating band alignments
Bulk calculations alone are insufficient to provide band alignments, since they contain no absolute reference for the electrostatic potential. The procedure therefore consists of two separate calculations: i) a bulk calculation to obtain the bulk band structure relative to the average electrostatic potential; and ii) a slab calculation to obtain the difference between the average of the electrostatic potential in the bulk and in vacuum.
The accuracy of the scheme was evaluated by investigating the following issues: the influence of different XC functionals, the difference between the m- and a-plane orientations, and the effect of relaxing the surface layers.
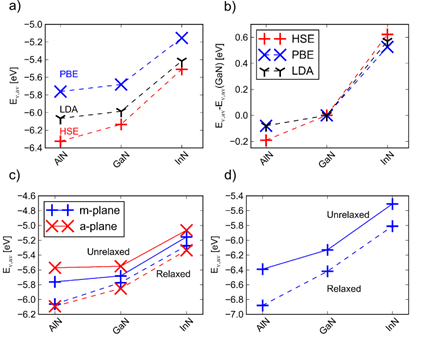
Figure 1 a) Valence-band (VB) positions
(Ev,av) b) Same
data as in a), but expressed relative to the GaN VB
position. c) The effect of relaxation on
the position of Ev,av. Calculated for
relaxed and unrelaxed surfaces d) The effect of
relaxation on the position of Ev,av.
Calculated for relaxed and unrelaxed surfaces of AlN, GaN, and InN
using HSE06. Electron affinities and ionization potentials change
dramatically when calculated with HSE06 compared to LDA and PBE (Figure 1 a).
However, relative band alignments are less sensitive to the choice of
functional (Figure 1 b). Electron
affinities for the nonpolar m- and a-plane are
similar when relaxations are included (Figure 1 c). The effect of relaxation is larger within
HSE06 than with PBE (Figure 1 c and d). These
results have allowed us to propose the most efficient procedure to calculate
alloy band alignments.
We have also investigated water
oxidation reactions on surfaces of wurtzite nitrides
and oxides. By combining calculated onset potentials for water oxidation with
band alignments we can predict if a given material will be able to oxidize
water or whether a co-catalyst is needed. In electrochemical water oxidation
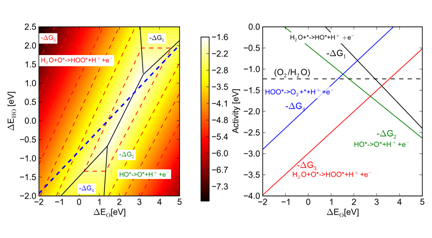
water is decomposed into electrons, protons and oxygen molecules:
2H2O -> O2 + 4(H+ + e-)
We consider the following one-electron
elementary reaction steps: 1) H2O
+ * -> HO* + (H+ + e-)
2) HO*
-> O* + (H+ + e-)
3) H2O
+ O* -> HOO* + (H+ + e-)
4) HOO*
-> O2 + (H+ + e-)+ *
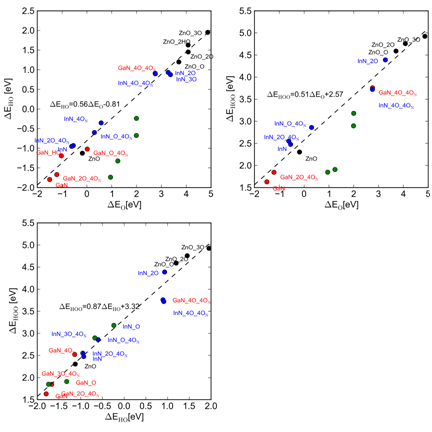
Based on detailed studies of ZnO, GaN and InN (0001) surfaces we
have established that certain linear correlations exist between the
intermediates in the water oxidation reaction (Figure 2).
These correlations allow us to express the reaction energies
of the elementary reaction in terms of the adsorption energy of just a few of
the intermediates. In this way we have constructed activity volcanoes
describing the minimum onset-potential needed to drive the photoelectrochemical
reactions (Figure 3). These activity volcanoes allow us to more quickly screen
new materials for water oxidation activity.
Figure 2 Top left: HO* binding
energies as a function of the O* binding energy. Top right: HOO* binding
energies as a function of the O* binding energies. Bottom: HOO* binding
energies as a function of HO* binding energies. In all cases we use the binding
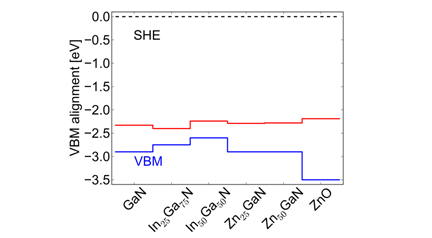
energy at the most stable adsorption site. Figure 3 Activity for water oxidation. Finally we combine band alignments with calculated onset
potential for a range of wurtzite nitride and oxide
compounds potentials (Figure 4). It is
clearly seen that all of these compounds may oxidize water with no assistance
from any co-catalyst.
Figure 4 Position of the valence-band maximum (VBM) and onset
potential calculated based on linear correlations. Summary
We have addressed two important issues with regards to the
water oxidation reaction on nitride and oxide surfaces. First we have
established a solid understanding of the methodology of calculating band alignments,
based on which we have given guidelines on how to calculate band alignments accurately
and efficiently. Secondly we have
investigated the water oxidation reaction and established linear correlations
between reaction intermediates, by which we have constructed activity
volcanoes. By combining band alignments and the activity volcanoes we have
predicted that a range of nitrides and oxy-nitrides are well suited for water
oxidation.
The grant has allowed PI to move into the highly active area
of photocatalysis and enabled him to start exploring
other interesting materials systems such as TiO2. For postdoc P. G.
Moses it has been an excellent opportunity to build up his knowledge of
semiconductors and surface physics and apply it to catalysis problems,
Onset potentials for water oxidation
Copyright © American Chemical Society