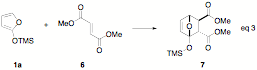AmericanChemicalSociety.com
Reports: B1 47559-B1: Diels-Alder Reactions of Silyloxy Furans: Scope and Limitations
Scott K. Bur, Gustavus Adolphus College
Progress:
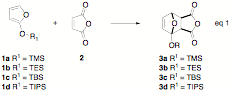
During this granting period, we expanded our study to include the reaction of a series of furans 1 with maleic anhydride at rt in the absence of solvent (eq 1). The size of the silyl R1 group was found to have a significant impact on the reaction; 1a reacts nearly instantaneously, 1b and 1c reacted over a few minutes, and 1d showed very little sign of cycloaddition, even after several days at rt. Interestingly, analysis of H1 NMR spectra of reaction mixtures involving 1a-c revealed near exclusive formation of the exo-adduct 3a-c.
We
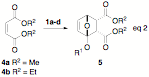
next turned our attention to various maleate esters to determine the effect of
the dienophile structure on the course of the reaction. In this series, the reaction of 1a with 4a,b
produced oxabicycles 5 resulting
from endo approach of the
dienophile (eq 2). While the
reaction of 1a with 2 produced 3a
almost immediately, use of 4a
required three days to go to completion, giving 5a (R1 = TMS, R2 = Me) as observed by NMR. In all cases we examined, the reaction rates corrolate with
the size of the silyl group in the furan; larger silyl groups reacted at slower
rates (Table 1). Based upon
coupling constants in the NMR spectra of the crude reaction mixtures, the endo-cycloadducts 5 were formed nearly exclusively.
Spectra taken at various time points throughout the reactions show no
equilibration between endo- and exo- adducts, though a fast equilibrium favoring the endo-adduct cannot be ruled out by these experiments, and
continuing progess of the reaction, not a rapidly established equilibrium
between reactants and products.
Table 1.a Entry Product R1 R2 %Yieldb 1 5a TMS Me 74 2 5b TMS Et 77 3 5c TES Me 69 4 5d TES Et 62 5 5e TBS Me 68 6 5f TBS Et 64 7 5g TIPS TIPS 55 8 5h TIPS TIPS 52 a) reactions run at 1 M in CDCl3 at rt. b) reactions followed for 3 d with the yield recorded relative to p-dichlorobenzene as an internal standard on day 3. Experiments
involving 1a and dimethyl fumarate
show a strong preference for placing the carbonyl proximal to the silyl group
in an endo orientation, providing
cycloadducts 7 as determined by
the analysis of coupling constants in the H1 NMR spectrum of the crude reaction
mixture (eq 3). The
nearly exclusive formation of the exo-adduct
3 was particularly surprising. This and the particularly large
difference in reaction rates as R1
increased in size led us to examine the reactions that form 3a and 5a
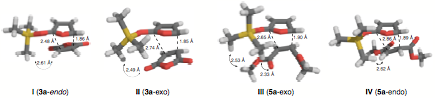
using density functional methods (B3LYP/6-31G(d)). The lowest energy transition states leading to both 3a and the corresponding endo-stereoisomer predicted that the endo-transition state I is 2.4 kcal/mol higher in energy than exo-transition state II (Figure 1). Similarly, the lowest energy transition states leading to
both 5a and the corresponding exo-stereoisomer predict that exo-transition state III is 4.6 kcal/mol higher in energy than the endo-transition state IV. In both
cases, the observed product distribution is consistent with that predicted by
the calculated energy differences.
All of the transition states modeled show a high level of stretch-mode
asynchronicity, where one of the forming bonds is significantly shorter than
the other. It is this
asynchronicity that gives rise to the reversal of diastereoselectivity. It is also clear how the size of the
silicon group influence the stereics of the transition states leading to
cycloadducts.
Studies
are currently underway to reduce each of the oxabicycles 3, 5,
and 7 to demonstrate the
synthetic potential this interesting diastereoselectivity has with respect
controlling the four contiguous stereocenters formed in the reaction.
Impact: This grant has opened up a new avenue of
research at Gustavus Adolphus College, and has attracted much student
interest. It has also provided the
PI several examples with which to enrich his teaching. The work accomplished under this grant
has provided the foundation for an NSF grant submission. During this grant period, the PI and a
student presented this work as a poster at the fall
2010 ACS meeting in Boston. Once
we have the results of the oxabicycle reduction experiments, a paper will be
submitted. On
an institutional level, this grant has been part of a successful effort to
increase undergraduate research opportunities in the summers. In combination with two institutional
grants (Merck Institute for Science Education and Howard Hughes Medical
Institute), the PRF grant has helped altered the research atmosphere at
Gustavus profoundly. Three
different students have been trained under this grant period. One student is applying to graduate
schools in chemistry, one is applying to medical schools, and one has become
interested in a research career.
Two of the three students currently trained under this grant period are
women.
Copyright © American Chemical Society