AmericanChemicalSociety.com
Reports: ND10 48813-ND10: Renewable Energy from a Neglected Photovoltaic Material: Boron Arsenide
Allen J. Bard, University of Texas (Austin) and Alan H. Cowley, University of Texas (Austin)
Synthesis of BAs
The synthesis of boron arsenide (BAs) with exact 1:1 boron:arsenic stoichiometry turned out to be more time consuming than originally anticipated. To a large extent, this was due to the relative volatility of elemental arsenic and the consequent production of materials with high boron:arsenic mole ratios. For example, the metathetical reaction of sodium arsenide with boron trihalides resulted in the formation of products with boron-to-arsenic mole ratios of approximately 4:3.
In an effort to overcome this problem, several single-source precursors with pre-formed boron-arsenic bonds were synthesized, characterized, and subjected to thermolysis in order to remove the ligands. Figure 1 shows an example of a precursor with ligands designed to leave when the precursor is treated with methanol. However, only arsenic deficient materials were isolated from such reactions.
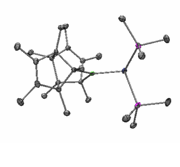 |
Figure 1. (C10H15)2BAs(SiMe3)2 |
A successful synthesis of boron arsenide was achieved via the thermal reaction of elemental boron and elemental arsenic in an evacuated sealed silica tube at 800oC. Interestingly, the 1:1 stoichiometry reaction of elemental boron and arsenic under similar conditions had been reported several years ago.1 However, in our hands the use of these particular reaction conditions resulted in a product with a 1:0.57 mole ratio of boron to arsenic. As shown in Table 1, subsequent experiments in which the reaction stoichiometry was varied revealed that it was necessary to use two molar equivalents of arsenic in order to produce the desired boron arsenide product. The identity of the boron arsenide was confirmed by X-ray powder diffraction (Figure 2) and X-ray photoelectron spectroscopy (Figure 3).
Table 1. Stoichiometry variations in the thermal reaction of elemental boron and elemental arsenic.
Arsenic mole equivalents of reactants | B:As mole ratio of products |
2 | 1:1 |
1.5 | 1:0.79 |
1.25 | 1:0.75 |
1 | 1:0.57 |
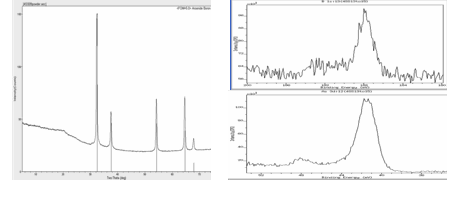 | |
| Figure 2. X-ray powder diffraction of BAs. | Figure 3. X-ray photoelectron spectroscopy of BAs. |
Photoelectrochemical Study of BAs
The goal of this part of the research project was measurement of the photoelectrochemical activity of the newly synthesized BAs, along with electrochemical characterization of this compound, in order to provide some guidance for the synthesis of more photoactive BAs materials.
For the electrochemical measurements, the newly synthesized BAs powder was dispersed in ethanol and placed on an F-doped tin oxide (FTO) electrode, followed by air drying, in order to prepare a thin film. The photoelectrochemical properties and activity were then tested in 1 mM methyl viologen (for photoreduction) or 10 mM Na2SO4 (for photooxidation) with a 0.2 M sodium phosphate buffer solution (pH 7). Various BAs materials prepared by different methods have been tested (as described earlier) and the BAs powder prepared with a mole ratio of 1:2 B to As was found to exhibit some photoactivity. This BAs material was subsequently shown to possess 1:1 stoichiometry on the basis of X-ray photoelectron spectroscopy (XPS) studies.
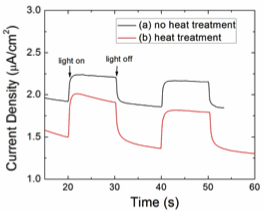
The electrochemical results (Figure 4a) showed that these as-synthesized BAs materials have p-type behavior (showing photoreduction) and the photocurrent was found to be ~ 0.3 µA/cm2 under full Xe lamp irradiation (intensity ~100 mW/cm2). The conductivity of BAs powder was compared with those of other oxide semiconductor powders such as TiO2 and BiVO4 and it was found that the as-prepared prepared BAs powder was 2 to 3 orders of magnitude more conductive than TiO2 or BiVO4 powder. This is probably due to too much doping of the BAs, therefore we are currently focusing on doping compensation with post treatment in order to decrease the doping level. The preliminary results showed that heat treatment at 380oC in air increased the photocurrent of BAs to ~0.5 µA/cm2 in 1 mM methyl viologen with a 0.2 M sodium phosphate buffer solution at an applied potential of -0.15 V vs Ag/AgCl (Figure 4b). Future work will focus more on optimization of the post treatment process for doping compensation such as using different dopants or controlling their amounts. We are also planning to optimize the BAs film preparation in terms of film thickness and the use of various electrodes.
Figure 4. (c) Current-time response curve of BAs films on an FTO electrode before (a) and after (b) heat treatment in 1 mM methyl viologen with 0.2 M sodium phosphate buffer solution (pH 7) at an applied potential of -0.15 V vs. Ag/AgCl with light chopping under Xe lamp irradiation (ca. 100 mW/cm2). Heat treatment was performed at 380oC for 5 min.
1 Perri, J. A.; LaPlaca, S.; Post, B. New group III-group V compounds: BP and BAs. ActaCrystallographica 1958, 11, 310.
Copyright © American Chemical Society