AmericanChemicalSociety.com
Reports: DNI5 49557-DNI5: Transport Kinetics of Surfactant Molecules between Emulsion Particles Probed by Surface Specific Second-Harmonic Generation
Elsa Yan, Yale University
Introduction
Emulsion is important in the petroleum industry, which is involved in crude oil transportation, refinery, oil cleanup, etc. In these processes, the emulsion formulism continuously changes. Hence, a fundamental understanding of the transport mechanism of surfactant molecules between emulsion particles is crucial to optimize the processes in order to increase the efficiency of petroleum production and develop more eco-friendly procedures.
To study the molecular transport process, we apply a second-order optical technique, second harmonic generation (SHG). Due to its coherent nature, SHG has the advantage of being surface-selective. It has been applied for investigating various chemical, physical, and biological phenomena at interfaces. Recently, SHG has been extended to study surfaces of colloidal systems, including clay particles, polymer particles, and emulsion.
Here, we take the advantages of the coherent nature of SHG to probe the transport kinetics of surfactant molecules between emulsion particles and aim to examine various factors that govern the transport process. The results will provide insight into improving the production processes in petroleum industry.
Experimental Methods
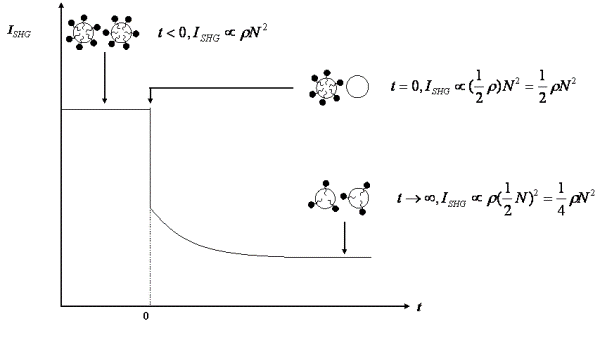
Figure 1. Time dependence of ISHG upon mixing equal volumes of donor and acceptor emulsion particles.
Due to the coherent nature of SHG, the SHG intensity (ISHG) depends quadratically on surface population of the adsorbed molecules on individual particles (N) but linearly on the particles density (r):
We measure the rate of detergent molecules transport from
donor to acceptor particles, where the acceptor particles are plain emulsion
and donor particles are emulsion doped with detergent probe molecules that provide
strong SHG signals. First, the SHG intensity is measured from the donor
particles at a particle density, r.
Then, an equal volume of acceptor particles at the same particle density is
injected at t = 0. Although the overall particle density stays the
same, the density of donor particles is halved. Hence, ISHG
becomes rN2/2. At t
> 0, detergent molecules transfer from the donor to acceptor particles
leading to a decay of ISHG until a new equilibrium is
established (t → ∞), where the detergent probe molecules are
equally distributed between the donor and acceptor particles. At equilibrium,
the particle density becomes r
and the surface population becomes N/2 such that ISHG
becomes rN2/4. Thus,
the SHG intensity at time t < 0, t = 0 and t → ![]() are in the
ratio of 1: 0.5: 0.25 (Fig. 1) and the decay of the SHG signal reveals the
kinetics of detergent transport between emulsion particles.
are in the
ratio of 1: 0.5: 0.25 (Fig. 1) and the decay of the SHG signal reveals the
kinetics of detergent transport between emulsion particles.
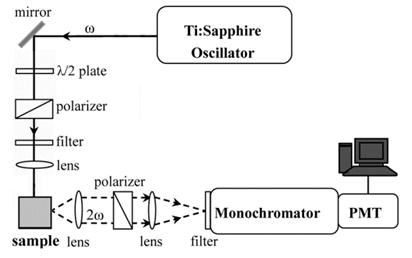
The SHG system contains a Ti:sapphire oscillator (MaiTai, Spectro-Physics), at 800 nm with an 18 mW output and a repetition rate of 5 kHz (Fig. 2). The SHG signal at 400 nm was spectrally separated by a monochromator and detected by a photomultiplier tube (R4220P, Hamamatsu).
Figure 2. The SHG setup.
The oil/water emulsion was prepared by mixing 5mM 1-dodecanol in n-tetradecane (Aldrich > 99%) with 5mM sodium dodecylsulfate (SDS) (Sigma > 99%) in water at a volume ratio of 1:9 followed by ultrasonication (MISONIX XL-2020) for 1 minute.
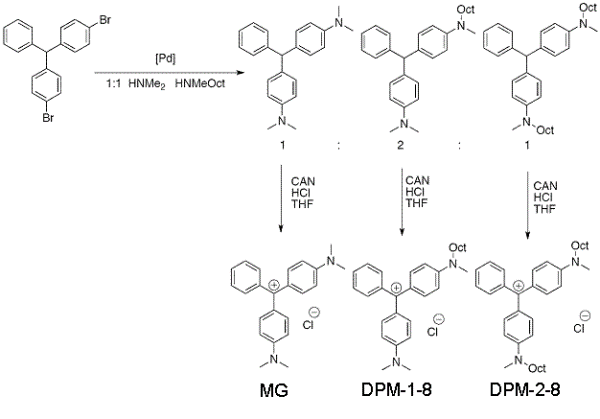
We synthesized (4-(dimethylamino)phenyl)(4-(methyl(octyl)amino)phenyl)(phenyl) methylium chloride (DPM-1-8) and used it as a detergent probe molecule. It contains a charged headgroup similar to malachite green (MG), which has an absorption at ~400 nm in resonance with the second-harmonic frequency and has proven to be effective in generating SHG signals on emulsion surfaces. The synthesis was done by using the Buchwald-Hartwig coupling of 4,4'-dibromo-triphenylmethane with equimolar quantities of dimethylamine and methyloctylamine afforded a statistical mixture of bis-aminated triarylmethanes, which were separated by flash chromatography. 4-((4-(dimethylamino)phenyl)(phenyl)methyl)-N-methyl-N-octylaniline was oxidized by cerium (IV) ammonium nitrate, in the presence of hydrochloric acid, as shown in Fig. 3. The synthesis yielded MG, DPM-1-8 and bis(4-(methyl(octyl)amino)phenyl)(phenyl) methylium chloride (DPM-2-8) for the SHG experiments.
Figure 3. Synthesis of the detergent probed molecules.
Results and Discussion
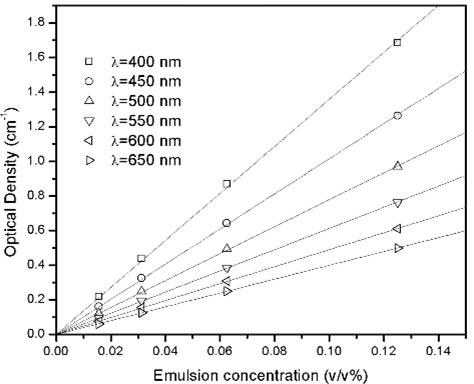
Figure 4. The UV-visible absorption measurements of emulsion.
Characterization of Emulsion
The emulsion was prepared and diluted to various concentrations for the UV-visible absorption measurements (Fig. 4). The absorbance at the selected wavelength was plot against the emulsion concentration, yielding a linear dependence as shown in Fig. 4. These results suggest the emulsion particles were mono-dispersed. The diameter was calculated to be 264 ± 15 nm.
Transport kinetic of surfactant molecules
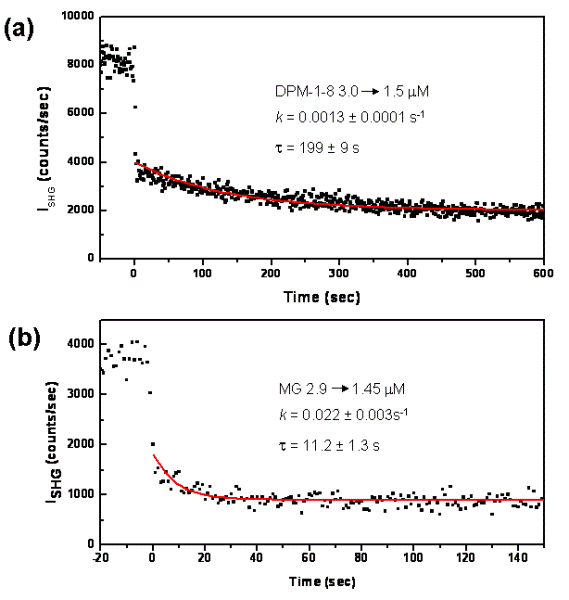
The transport rate of DPM-1-8 was measured. At t <
0, the emulsion was mixed with DPM-1-8 to yield a final particle density at 8.1x109 particles/cm3 and
a final DPM-1-8 concentration at 3.1 mM.
At t = 0, an equal volume of plain emulsion at the same density was
injected and the SHG intensity was monitored. Fig. 5(a) shows the
time-dependence of the SHG intensity, which is in agreement with the
theoretical predicted intensity at t < 0, t = 0 and t
→ ![]() in
the ratio of 1:0.5:0.25. The normalized SHG intensity at t > 0 is
fitted into the equation,
in
the ratio of 1:0.5:0.25. The normalized SHG intensity at t > 0 is
fitted into the equation,
to yield the transport rate constant, k = 0.0013 ± 0.0001 s-1. The experiment was repeated with MG, which lacks the octyl-tail (Fig. 3). The rate constant of MG transport was found to be 0.022 ± 0.003 s-1 under the same conditions (Fig. 5b). The transport rate for MG is 18-time faster than that for DPM-1-8. The result supports that the SHG method can be applied to probe transport kinetics of detergent molecules between emulsion particles.
Figure 5. The time-dependence of the SHG intensity.
Conclusion and Future Directions
In this study, we have demonstrated that SHG is an in situ tool to measure rates of detergent transport between emulsion particles. We will further apply the method to investigate (1) the role of hydrophobicity of detergent by synthesizing the detergent probe molecules with different number or length of carbon chains, (2) the transport mechanism by changing the particle density, oil phase, and electrolyte concentration, and (3) the kinetic parameters by varying the temperature to obtain the activation energy and pre-exponential factor. The results will reveal the determining factors that control detergent transport between emulsion particles, which will provide insight into optimizing the chemical processes carried out not only in petroleum industries, but also in pharmaceutical industries.
Copyright © American Chemical Society