AmericanChemicalSociety.com
Reports: G5 47027-G5: Rational Self Assembly of Macromolecular Arrays for Optimized Light Harvesting and Photocatalytic Hydrogen Production
Jonas I. Goldsmith, Bryn Mawr College
The research supported by this grant has progressed in several directions during the last year. The funds from this grant have been used to pay summer salary for the PI as well as to provide some support for materials and supplies. The support of students was all paid for by Bryn Mawr College. The major accomplishments during this period have included the synthesis of ligands and transition metal complexes (TMCs) as well as the development of photoelectrochemical methods to examine the generation of the active, reduced, transition metal complexes necessary for the reduction of hydrogen to water.
1. Synthesis of new ligand for functionalizing graphene-based nanostructures with transition metal catalysts for light harvesting
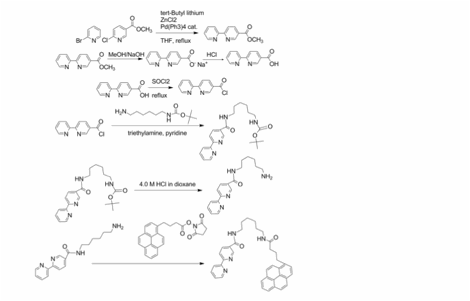
In addition to colloidal metal nanoparticles, carbon-based nanostructures such as single walled carbon nanotubes (SWNTs) are interesting candidates for charge collection in light harvesting systems. Previous work in my research group has demonstrated the functionalization of SWNTs by pyrene terminated cobalt (II) terpyridine complexes and, to extend the functionalization methodology described in this grant to carbon nanotubes the bipyridine pyrene ligand below has been designed and synthesized. This ligand will allow the synthesis of a wide variety of TMCs with both the necessary photophysical properties for light harvesting (note that the analogous metal terpyridines are not good candidates for light harvesting due to their relatively short excited state lifetimes) and a functional group (the pyrene) that will facilitate adsorption on the SWNT surface.
Figure 1. Synthesis of bipyridine-pyrene ligand. 2. Development of
multi-metallic complexes for light harvesting While the final
goal of this project is to create colloid-supported macromolecular light
harvesting assemblies, the behavior of such systems containing many metal
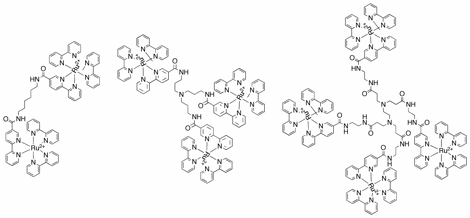
centers is best understood by degrees. To that end, a series of ligands and transition metal complexes containing 2, 3 and
4 metal centers has been synthesized (see Figure 2 below). Electrochemical
techniques including cyclic voltammetry at a rotating
disc electrode as well as collection experiments utilizing a rotating ring-disc
electrode have been utilized to characterize the redox
properties of these molecules and they will be shortly used as photosensitizers in light harvesting hydrogen production
systems. The goal of this work is to understand the effect of clustering the
light harvesting centers in close proximity (as would be the case in
colloid-supported macromolecular systems) in order to facilitate judicious and
thoughtful design of these systems.
Figure 2. Multimetallic ruthenium
(II) tris-bipyridine complexes. 3. Photo-induced chronoamperometry (PICA) to probe the efficiency of light
harvesting and electron transfer One of the major
goals for this project was to examine vectorial
electron transfer in self-assembled monolayers of
light-harvesting chromophores. This is not a trivial
problem and the methodology that we have developed may be able to probe such
systems in a deep and fundamental way. Merely applying standard electrochemical
techniques to adsorbed monolayers can give some
assessment (either using a Tafel methodology for
potential step experiments or via the techniques of Laviron for fast scan cyclic
voltammetry) of electron transfer kinetics. However,
the real scenario that we are interested in is one in which a photogenerated excited state undergoes electron transfer
quenching. To examine such a system, we have developed a technique that combines
chronoamperometry with a pulsed LED excitation source
(see Figure 3 for a picture of the PICA apparatus).
Figure 3. PICA
apparatus/system. The light pulse from the LED generates the excited photosensitizer
which is quenched by a sacrificial reductant
present in the solution. This generates the reduced photosensitizer,
which is the active species that provides the reducing equivalents necessary to
reduce protons to molecular hydrogen. The experiment is carried out in an aprotic environment so, instead of reducing protons, the
reduced photosensitizer is re-oxidized by a working
electrode held at the appropriate potential, and the current from that process
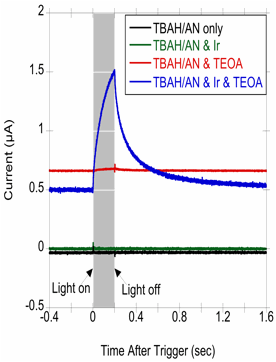
is measured. The result is a current transient, as can be seen in Figure 4,
that is only present when all components of the system are in place. Without
either the photosensitizer or the sacrificial reductant, no current is observed. From the decay of the
current transient after the end of the light pulse, the concentration of the
reduced photosensitizer species generated by the
light pulse can be calculated. The intensity and duration of the light pulse
can be modulated (i.e. the photon flux can be controlled) and the efficiency of
light harvesting can be determined by comparing the amount of reduced photosensitizer formed to the number of photons absorbed by
the solution.
Figure 4. PICA
experiments for solutions containing
a)
Solvent and supporting electrolyte (TBAH/AN
only)
b)
Solvent, supporting electrolyte and photosensitizer (TBAH/AN& Ir)
c)
Solvent, supporting electrolyte, sacrificial reductant and no photosensitizer
(TBAH/AN & TEOA)
d)
Solvent, supporting electrolyte, sacrificial reductant and photosensitizer
(TBAH/AN & Ir & TEOA)
This technique has been validated on a variety of solution-based
systems and its implementation for systems involving self-assembled monolayers is currently underway. For monolayers,
the experiments will be largely the same. An electrode with a layer of photosensitizer will be placed in a solution containing the
sacrificial reductant and will be photoexcited.
Reduced photosensitizer molecules that result will be oxidized by the electrode and the resulting current
will be measured, indicating how effectively reducing equivalents can be
transferred from the photosensitizer to the electrode
surface. As the final goal of this project involves functionalized metal nanoparticles serving as charge reservoirs for photochemically generated reducing equivalents, conducting
PICA experiments on electrodes functionalized with photosensitizers
will give valuable insight into how such systems might behave.

Copyright © American Chemical Society