AmericanChemicalSociety.com
Reports: AC2 47994-AC2: Assessing the Potential of B/Ca in Planktonic Foraminifera as a Proxy of Seawater pH: a Sediment Trap Calibration
Yair Rosenthal, Rutgers University
Objectives and work progress
The primary objective of this proposal is to test the utility of planktonic foraminiferal boron-to-calcium (B/Ca) ratios as a proxy for seawater pH using samples collected from Bermuda Time Series sediment traps over the past >20 years. It has been hypothesized that both temperature and pH (or CO3 ion concentration) affect the incorporation of B into the calcitic foraminiferal shells. Therefore, we need to quantify the dependence of the partition coefficient of B on both parameters in order to be able to reconstruct paleo-pH.
Our work over the past year has focused on the following issues:
1. Sampling of sediment traps material
During the first year we focused on samples from two stations namely i) Bermuda and ii) South China Time Series.
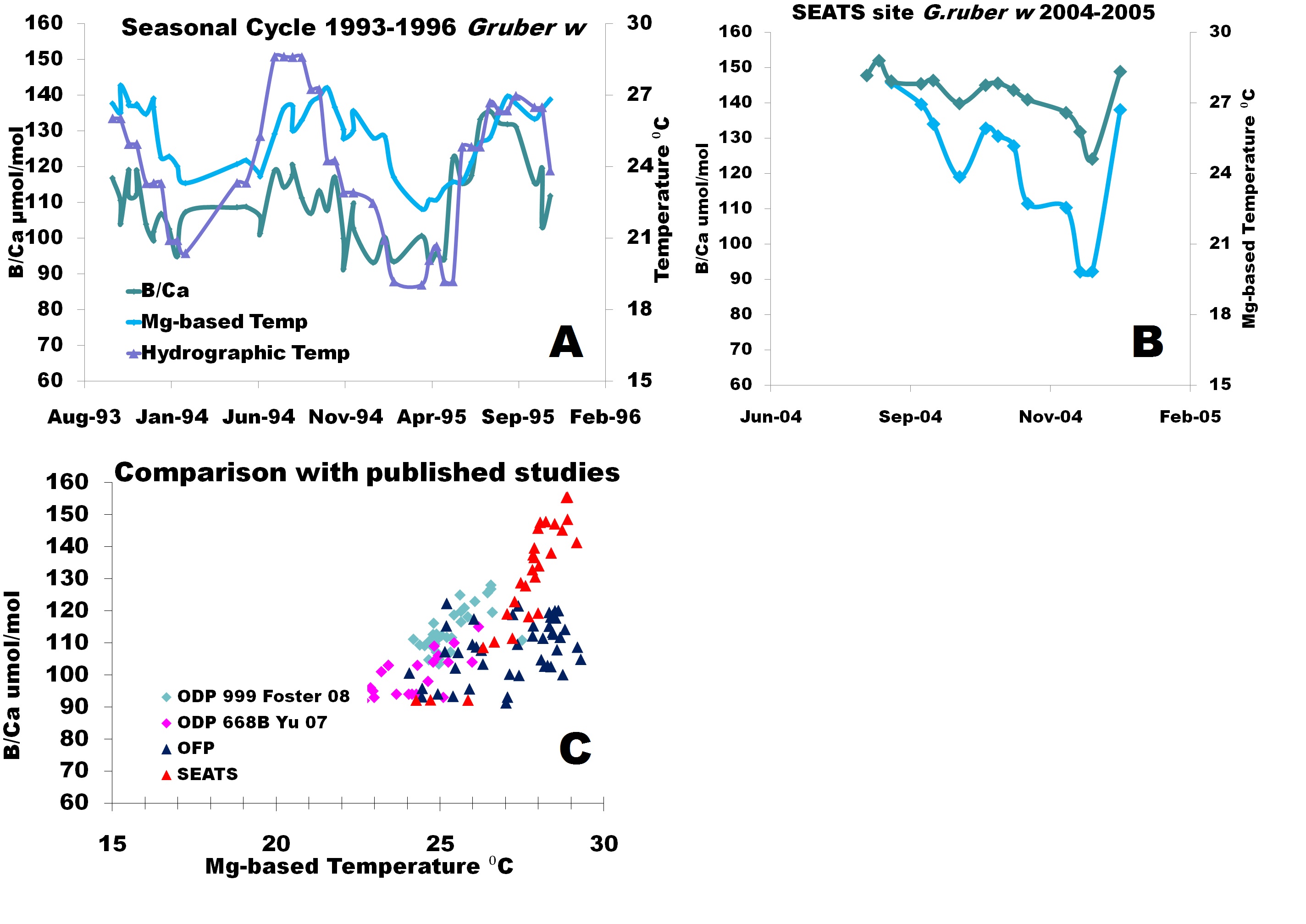
Graduate student Tali Babila has extended the Bermuda sediment trap calibration and completed the sampling of 8 full annual cycles from 1985-1987, 1993-1996 and 2006-7 in two week and bimonthly resolution. We sampled four planktonic foraminifera (G. ruber, G. sacculifer, P. obliquiloculata and N. dutertrei) but so far our work focused only on G. ruber. Our initial analysis has indicated that B/Ca varies with shell size. Consequently, we have divided each sample into discrete size fractions that will be analyzed separately. The 1993-1996 data (Fig 1A) show that B/Ca increases during warm sea surface temperatures (SSTs), when pH is lowest and decreases during colder SSTs following the seasonal cycle. As surface water pH at this site is largely driven by temperature determining the relative contributions of these parameters is critical. We also completed the analysis of samples from the South China Sea traps (SEATS). These samples cover 5 months interval in 2004 (G. ruber ) (Fig. 1b). These results are also compared with data obtained from the underlying surface sediments to assess the compatibility between fresh and fossil material.
Comparison of our data and published results (Fig 1c) confirms a positive correlation between temperature and boron incorporation into foraminiferal calcite. The Bermuda B/Ca results are, however, less sensitive to temperature possibly due to the additional influence of pH. To determine the sole dependence of pH on B/Ca, boron isotopic measurements (in collaboration with Dr. Pallavi Anand) will be made as an independent proxy for pH so that B/Ca can be directly calibrated to calcification pH. This approach will allow for both the temperature and the pH effect on boron incorporation to be determined.
2. Sediment studies
To test the reliability of B/Ca in fossil material we have also measured B/Ca in core-tops planktonic foraminifera from different ocean basins. Results obtained on core-tops samples suggest that there is no diagenetic alteration of the primary B/Ca signal in the planktonic shells. The core-top calibrations are generally in accord with the sediment traps.
The data collected so far already shed new light on the geochemical controls on B/Ca in planktonic foraminifera. Previous studies have debated whether temperature plays a substantial role in the incorporation of B into the calcitic shells with opposing results from these studies. Our data suggest that overall there is a temperature influence on B incorporation although within each region there might be additional, hitherto not well understood, local effects (in addition to pH). The results suggest that the initial assumptions of temperature and pH control might not be justified and a new approach is needed. We are now exploring other directions and will repeat these studies using other foraminiferal species.
Personnel and budget
While several students have been involved in this project, it is becoming the thesis subject of PhD candidate Tali Babila. Tali passed her qualifying exams in 2009 and since then has turned her full attention to the project. As the sample preparation for these analyses is very laborious I have used these funds to support other students in helping her in these project. Tali has discussed her results in two meeting this summer. First in an NSF supported meeting on ocean acidification in Catalina Island (CA) and second, at the International Conference in Paleoceanography held in San Diego.
Abstracts and manuscripts in preparation partially supported by this grant
Babila, T, Huang, K-F, Rosenthal, Y, Conte, M.H and Lin H.-L (2010). Development of B/Ca as a seawater proxy using sediment trap time series. Poster at International Conference of Paleoceanography, San Diego CA
Babila, T. (2009). Development of B/Ca in planktonic foraminifera as a proxy for
seawater pH. Poster at Urbino Summer School of Paleoeanography, Italy
Babila T, Field MP, and Rosenthal Y. (2009). Determination of B/Ca in planktonic foraminifera as a proxy for seawater paleo-pH. 8th International Sector Field ICPMS Conference in Ghent, Belgium.
Huang, K-F, Rosenthal, Y. et al., (pending submission). Reconstruction of change in surface water pH and pCO2 in tropical South China Sea using foraminiferal multi-proxies approach.
Figure 1: (Provisional data) (A) Preliminary B/Ca µmol/mol (green) results for G.ruber white from biweekly resolution from 1993-1996. Contemporaneous hydrographic sea surface temperature (purple) from the nearby Bermuda Atlantic Time Series (BATS) station and Mg/Ca based calcification temperature (blue) using previously published G. ruber calibration from OFP (Anand et al. 2003); (B) Monthly B/Ca µmol/mol (green) and Mg/Ca based calcification temperature (blue) results for G. ruber white from August to January 2004 from the South East Asian Time Series (SEATS); (C) Sediment trap and core top samples from South China Sea (this study) compared with published results. The new data show general temperature control on B/Ca in the surface water species G. ruber but with a very large scatter suggesting additional influences on B/Ca.
Copyright © American Chemical Society