AmericanChemicalSociety.com
Reports: G1 47539-G1: Harnessing the Reactive Intermediates of Catalytic-Heterofunctionalization
Aaron Aponick, University of Florida
With the support of this ACS-PRF grant we have made significant progress in the discovery of new dehydrative reactions of unsaturated alcohols. Our work has been focused on developing allylic and propargylic alcohols as electrophiles in gold-catalyzed processes whereby a tethered nucleophile adds to the pi-bond with concomitant loss of water (Scheme 1). This is a new mode of reactivity in the burgeoning area of homogenous gold catalysis, which enables the preparation of a variety of ubiquitous structural motifs and as such should find use in total synthesis.
Scheme 1. Au-catalyzed dehydratve cyclization reactions.
At the
outset, it was our intention to search for new catalytic
heterofunctionalization reactions of alkenes that proceed under mild reaction
conditions and would be suited for use in complex molecule synthesis. Through a series of preliminary
experiments, it was found that allylic alcohols are extremely reactive towards
gold catalysts. When substrates
containing an allylic alcohol tethered to a nucleophile were treated with 1 mol
% Ph3PAuCl and 1 mol% AgOTf in methylene chloride, a clean
cyclization smoothly proceeds with elimination of water. Careful control
experiments demonstrated that both the gold and silver complexes are necessary
for the reaction. Further studies were designed to probe the effects of
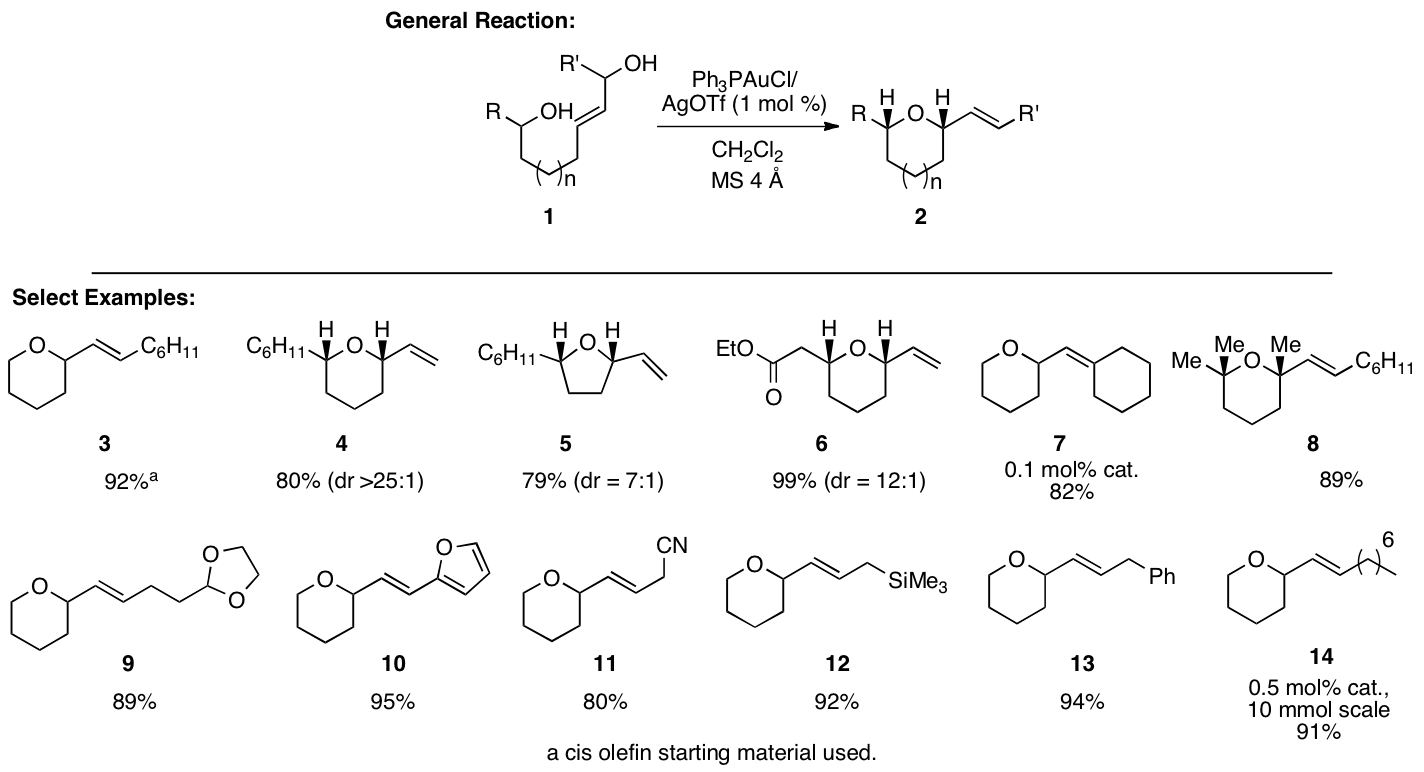
substitution on the allyl moiety and the results are shown in Figure 1. A variety of substrates performed well
in the reaction including cis
olefins, aldol adducts, tertiary allylic alcohols, tri-substituted olefins
(forming a quaternary stereogenic center), and other assorted functional
groups. The reaction proceeds in
high yield in all cases with high cis
selectivity when applicable. In
addition, the reactions could be performed at temperatures as low as -78 °C and
the catalyst loadings could be reduced to 0.1 mol % without negative
consequences.
Based
on these results, the analogous propargylic diols would be predicted to undergo
similar reactions, although the products produced would be allenol ethers. We reasoned that these reactive
intermediates could be utilized in an efficient process if an additional
alcohol were present in the molecule, and would form unsaturated spiroketals
such as those contained in natural products like spirastrellolide A and okadaic
acid. The reaction sequence would
be predicted to proceed from the triol 15
to the spiroketal 17 via the allenol
ether 16 (Scheme 2). In principle, both processes could be
catalyzed by a cationic metal. This
route was particularly attractive because the olefin would be precisely placed according
to the general scheme leaving no ambiguity in the sizes of the rings formed,
which is often a challenging issue.
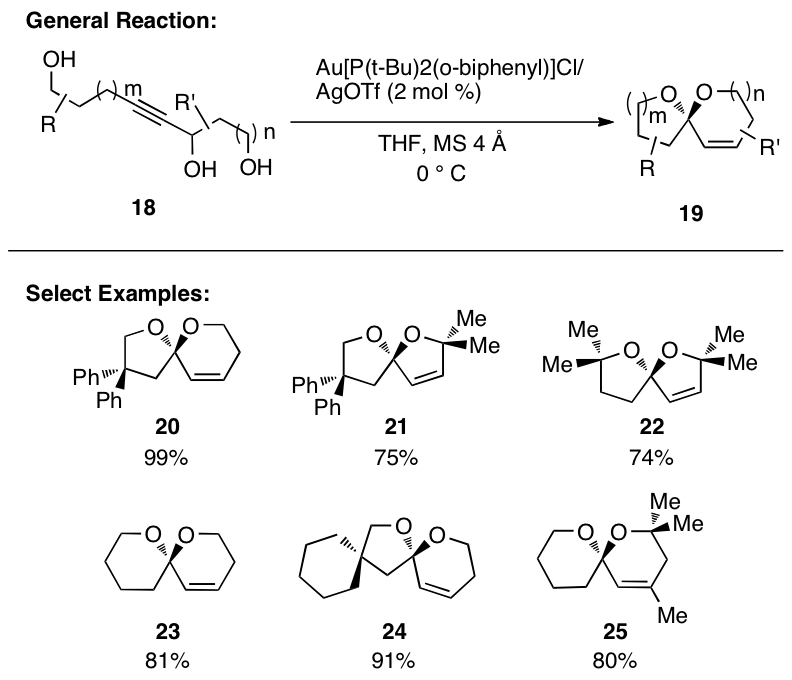
Gratifyingly,
this reaction sequence works extraordinarily well, smoothly generating the
desired products in high yield (Figure 2). Interestingly, the reaction functions best when Au[P(t-Bu)2(o-biphenyl)]Cl is employed with AgOTf in
THF at 0 °C. The loading can be
reduced to 2 mol% with reaction times ranging from minutes to just less than
1.5 h. Control experiments suggest
that Au[P(t-Bu)2(o-biphenyl)]OTf is the active catalyst
and rule out the possibility that the reaction is catalyzed by TfOH.
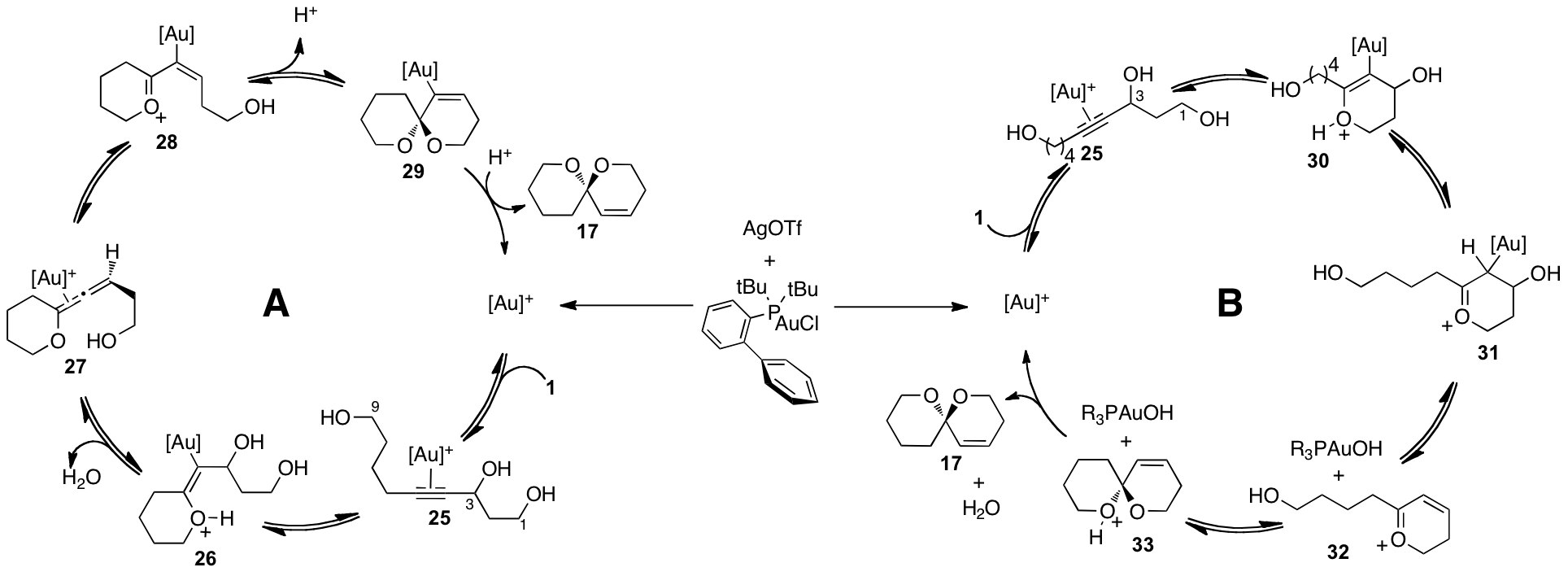
A likely
catalytic cycle for the reaction is presented in Scheme 3. Experimental evidence using the diol
generated by protection of the C1 or C9 hydroxyl group suggests that either the
C1 or C9 alcohol may cyclize first but both events eventually lead to the same
product.
This proposal
lead to the idea that the dual catalytic cycles could function independently
and provide unique reaction pathways.
If cycle B were to be accessed with substrates devoid of a second
nucleophilic alcohol, a different mechanism for further reactions from 31 would be necessary. To address this, heteroatom substituted
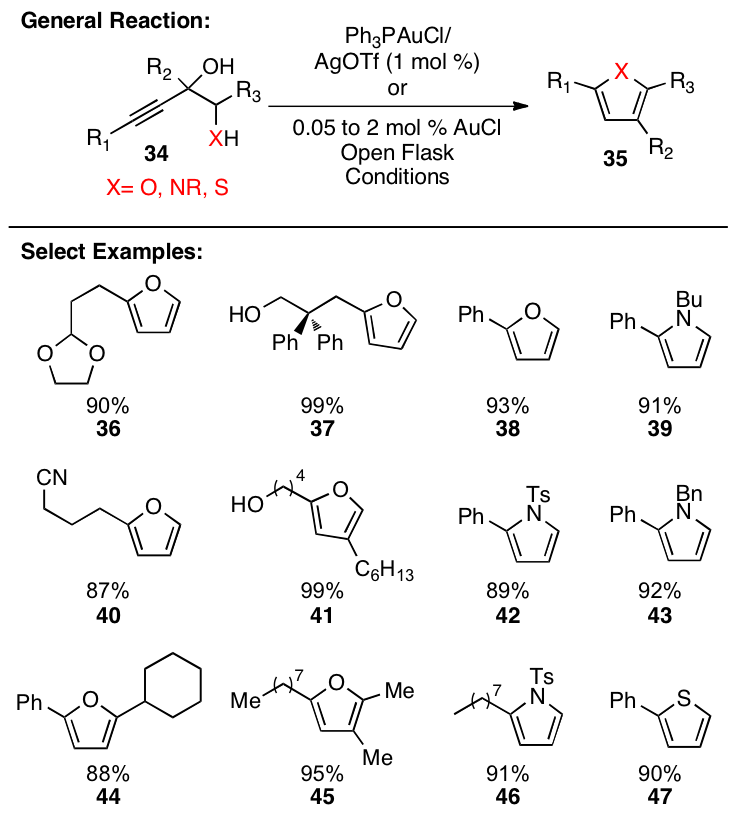
substrates such as 34 were prepared
and treated under the same reaction conditions (Figure 3). These systems rapidly aromatize to form
5-membered heteroaromatics in high yield.
Additionally, it was found that AuCl could be used as catalyst in an
open vessel without taking any precautions to exclude air or moisture. The reaction is very simple to perform
(essentially no precautions are necessary), rapid, high-yielding, exceptionally
clean (no additional product purification is generally necessary), and the
catalyst loadings are exceedingly low (as low as 0.05 mol%). Efforts are
currently underway to utilize catalytic cycle A (Scheme 3).
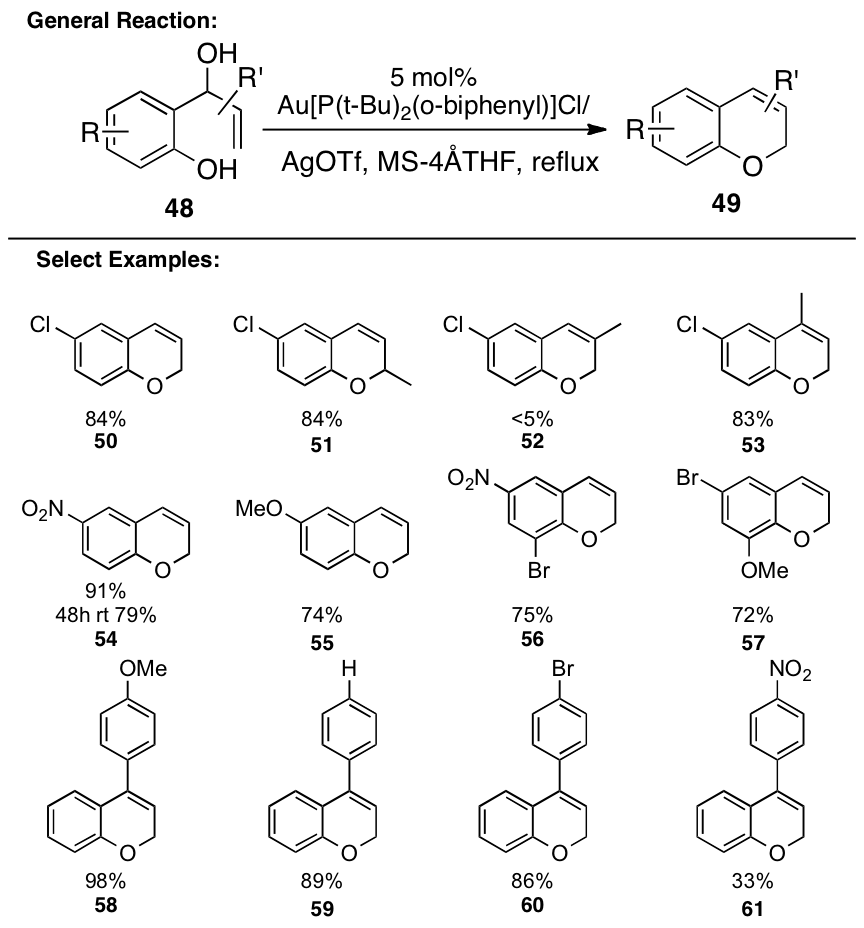
Since
these aromatic heterocycles were easily prepared, we sought to explore the synthesis
of additional heterocycles, namely 2-H-chromenes. In the process, it was found that endo-cyclization reactions
of allylic alcohols can also be catalyzed by Au-salts, but the conditions
required are much more forcing (Figure 4). Starting from substituted benzaldehydes, a wide variety of
differentially substituted chromenes are readily available in two steps.
The
results outlined above demonstrate that unsaturated alcohols undergo a variety
of dehydrative transformations under extremely mild conditions. These methods enable facile preparation
of highly useful cyclic ethers, monounsaturated spiroketals, and five-membered
heteroaromatics from readily prepared diols and triols. The reactions are rapid and generally
high yielding providing a concise synthesis of functional building blocks in
short order. Additional reactions
of this ilk are being developed and will be reported in due course.
Copyright © American Chemical Society