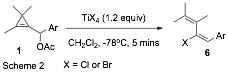AmericanChemicalSociety.com
Reports: GB1 45092-GB1: Synthesis and Transition Metal-Catalyzed [3+2] Cycloadditions of Methyleneaziridines
Christopher J. T. Hyland, California State University (Fullerton)
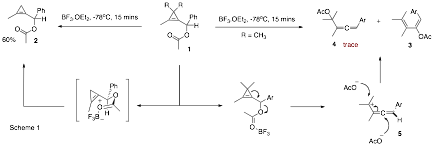
In the first year of this grant a side-project investigating the reactivity of cyclopropenyl acetates, which was related to the main investigation of methyleneaziridines provided a new and exciting avenue of investigation. Cyclopropenyl acetates are interesting building blocks for organic synthesis due to their inherent strain and the presence of binding sites for both p-philic and s-philic Lewis acids. It was initially found that in the presence of the s-philic Lewis acids BF3.OEt2 rearrangement of cyclopropene 1 to heteroatom substituted methylenecyclopropane 2 occurred (Scheme 1). Interestingly, the presence of a gem-dimethyl group on the cyclopropene ring led to the formation of Z-acetoxy-diene 3 with high stereoselectivity. The formation of diene 3 was accompanied by trace amounts of allene 4, which lends support to the intermediacy of an allenyl cation 5.
Further, screening of Lewis-acids
led to the discovery that TiCl4 causes cyclopropenyl
acetates 1 to rearrange to (E)-chloro-dienes
6 (Scheme 2). Again, this rearrangement
is thought to proceed via an allenyl cation 5, which is then intercepted by
chloride from the least hindered face. Work this year has completed the
substrate scope investigation for this reaction, which has been shown to be
general for a range of aryl groups. Interestingly, for alkyl-derived substrates
the E-selectivity drops
significantly. In addition, we were
delighted to find that TiBr4 can also mediate the reaction, allowing
stereoselective preparation of E-bromodienes.
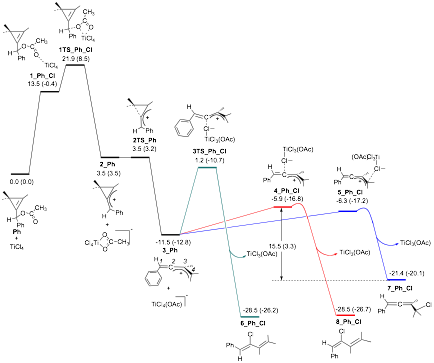
This year we have also worked
collaboratively with a computational chemist to elucidate the exact mechanism
of the halo-diene forming reaction. These studies
were in-line with out initial mechanistic thoughts, but provided additional
detail as well as an explanation for the surprising regioselectivity
of the halo-diene forming reactions (Figure 1).
These theoretical investigations uncovered
two important facts. Firstly, the complete stereoselectivity
of the reaction is likely due to the bulky TiCl4(OAc)- rather than Cl-
attacking the least hindered face of allenyl cation 3_Ph.
Secondly the selectivity for the reaction of diene 8_Ph_Cl (6 in Scheme 2) over allene 7_Ph_Cl is due to thermodynamic
control. Attack of TiCl4(OAc)- at C-4 of allenyl
cation 3_Ph
is reversible whereas attack at C-2 is irreversible, resulting in diene 8_Ph_Cl
being the only observed product.
Copyright © American Chemical Society