AmericanChemicalSociety.com
Reports: DNI5 48780-DNI5: Near-Field Vibrational Spectroscopy and Imaging of Chemical Species on Individual Nanoparticles During Catalytic (De)Hydrogenation
Michael J. Gordon, PhD, University of California (Santa Barbara)
1. Introduction
The goals of this PRF DNI project are to (1) develop a hybrid scanning probe microscopy system which combines atomic force imaging of surfaces with near-field vibrational spectroscopy for local identification of interface chemistry, (2) realize catalytically-active nanoparticles <10 nm with controlled shapes and compositions using solution and plasma-based methods, and (3) investigate catalytic hydrogenation reactions using the aforementioned equipment and materials. During the first year, grant funds were used to partially support two PhD graduate students in Chemical Engineering as well as purchase equipment, chemicals, and consumables for catalysis experiments. In particular, the project has seen advancement in several areas: (1) proof-of-concept experiments demonstrating near-field vibrational spectroscopy on graphitic surface species and hematin molecules, (2) successful synthesis of shaped Pt and Pd nanoparticle catalysts using growth promoters and microplasma jet discharges, and (3) initial catalytic testing of synthesized materials for selective hydrogenation of acetylene.
2. Experimental results
(a) Chemical imaging of surfaces
Our work on the development of a combined optical and atomic force
microscope for chemical imaging of surfaces continued during year 1 of
the PRF grant. The built-from-scratch system, show in Fig. 1, is now
operational for topographic AFM imaging, confocal optical
imaging/spectroscopy, and tip-enhanced
optical experiments. The basis of the instrument (and chemical imaging)
is localized vibrational spectroscopy made possible by enhanced optical
fields near the probe tip; in particular, Raman scattering [inelastic
light scattering due to the excitation of molecular vibrations] from
molecules in the tip-surface gap is enhanced due to plasmonic coupling
of light with the probe tip , providing a spectroscopic fingerprint of
chemical moieties on the surface. Figure 2 shows a topographic image of
Pt nanoparticles (see section 3b) imaged with the
hybrid system. To test the tip-enhanced functionality of
the system, a graphitized polymer surface was imaged with the probe tip
far-away vs. close (i.e., engaged with nN forces) to the
surface. As shown in Fig. 3, Raman signatures of the D and G-bands of
graphitic carbon can be seen only when the probe tip is engaged on the
surface - confirming the ability to locally identify surface chemistry.
Additionally, the system was used to identify hematin molecules (the
oxygen binder in red blood cells) on a surface in scanning tunneling
mode; again, enhancement of the Raman scattering only occurs when the
tip is within a few nm of the surface (Fig. 4). Experiments are
currently underway to construct chemical images of catalytic
particles/surfaces using the tip-enhanced functionality and scanning
probe capabilities of the system.
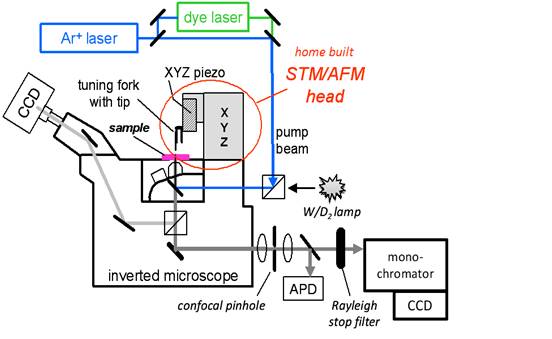
Figure 1: Hybrid
optical/scanning probe microscopy system for nanoscale chemical imaging.
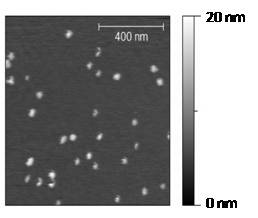
Figure 2: Pt
nanoparticles
imaged with the microscope system in fig. 1.
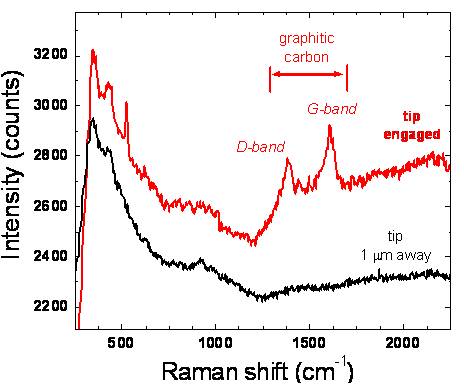
Figure 3:
Tip-enhanced Raman identification of graphitic surface species using
AFM mode.
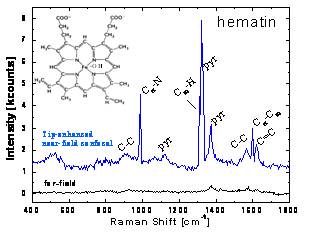
Figure 4:
Tip-enhanced Raman spectrum of hematin molecules on ITO surface using
STM mode.
(b)
Nanoparticle
synthesis
Shaped Pt nanoparticles (e.g. cubes, cuboctahedra, and octahedra) in
the 5-10 nm size range were successfully synthesized using
PVP (polyvinylpyrrolidone) surfactants and Ag as a growth promoter
using the reaction scheme in Fig. 5. Particles were subsequently
supported on colloidal SiO2 and calcined in O2/H2
atmospheres to remove
the PVP surfactant and studied via TEM, XRD, IR spectroscopy, and
catalytic testing. As an alternate method, Pd and SiO2-supported
Pd
nanoparticles
(Fig.
6)
were
synthesized
using DC microplasma jets seeded with
organometallic precursors. The resulting particles were size classified
using differential mobility analysis (confirming their tight size
distribution) and deposited on various surfaces for CO
adsorption experiments and catalytic testing.
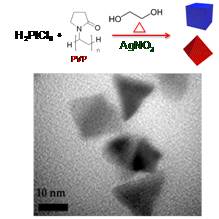
Figure 5: Reaction
scheme for shaped Pt nanoparticles and TEM image of synthesized
cuboctahedra.
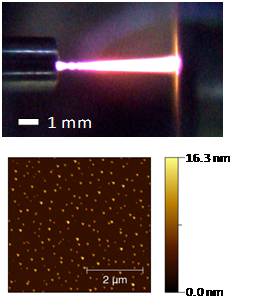
Figure 6: Microplasma discharge and AFM image of synthesized Pd nanoparticles.
(c) Catalytic experiments
The aforementioned Pt and Pd nanoparticles were tested for their
hydrogenation activity and selectivity using acetylene/H2
mixtures in a plug flow reactor as a benchmark for further
hydrogenation studies with more complex olefins and aromatics. As can
be seen in the light-off curves in Fig. 7, Pt nanoparticles with preferential [111]
crystal faceting (i.e., those with ~20% Ag growth promoter, namely
[cubo]octahedra) favored selective conversion of acetylene (C2H2)
to ethylene (C2H4) while particles with [100]
faceting (cubes with no Ag) favored total hydrogenation to ethane (C2H6).
Pd nanoparticles synthesized via microplasma also favored selective hydrogenation.
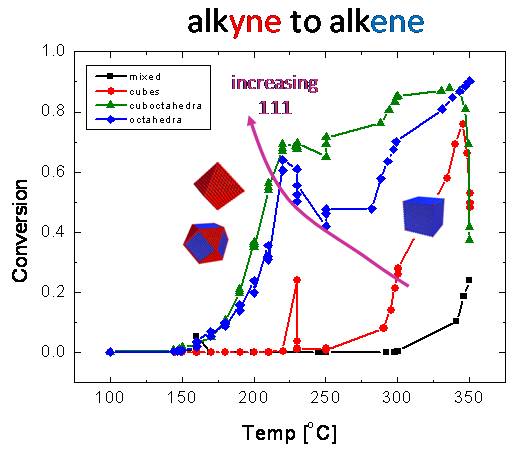
Figure 7: Light-off
curves for acetylene hydrogenation on different Pt nanoparticles
shapes. "Mixed" refers to particle synthesis using no shape
promoters.
3.Impact of research
Given the project's focus on theoretical and experimental aspects from nanoscience, optics, and chemical synthesis, the graduate students funded by the project are becoming experts in the fields of scanning probe microscopy, materials characterization, and catalysis. These students are working in a unique laboratory setting that provides interdisciplinary training, mentoring opportunities, and interactions with other students/post-docs. Three Chemical Engineering undergraduates also participated in the research; as a result of their accomplishments, two of these students received campus fellowships to continue research during the academic year and the third student spent a summer abroad on fellowship at the University of Cork in Ireland (this student is now attending graduate school).
The PRF-DNI grant has also had a substantial impact on the PI’s ability to acquire additional funding. Preliminary results made possible by this grant, specifically in the areas of microscope development and application work on catalytic surfaces and polymer solar cells, were recently integrated into an NSF-CAREER proposal that was awarded funding in Feb. 2010. In addition, the equipment development and experimental work in the last year has shown that nanoscale chemical imaging of catalytically-relevant surfaces and systems is not far away; in particular, the hybrid optical/scanning probe microscopy system, synthesis work, and catalytic testing supported by this PRF grant will soon allow us to probe and better understand how local effects influence chemical reactions and molecular transformations on surfaces. Future experiments with the tip-enhanced chemical microscope will focus on identification and imaging of adsorbates on the surfaces of Pt, Pt alloy, and Pd nanoparticles under a variety of conditions to investigate how size, crystal faceting, and surface alloying affect catalytic activity and selectivity.
Copyright © American Chemical Society

