AmericanChemicalSociety.com
Reports: DNI10 49002-DNI10: Tailorable Organic Nanoscale Piezoelectrics for Energy Harvesting
Geoffrey R. Hutchison, PhD, University of Pittsburgh
Background and Significance
Piezoelectrics are polar materials that produce electrical charge in response to a mechanical distortion, or change shape in response to an applied electric field. As such, they have achieved wide use as combined sensors and actuators for applications as wide-ranging as sensors, ultrasonic motors, scanning-probe microscopy manipulation, and airbag impact sensors. A variety of conventional piezoelectric bulk materials are known, including toxic perovskite materials such as lead zirconium titanate (Pb[ZrxTi1-x]O3 or PZT), poly(vinylidinedifluoride) (PVDF) and other semicrystalline polymers, quartz, and some liquid crystals. Previous work also has illustrated that layer-by-layer assembly of organic electrostatic polymers will yield piezoelectric activity, but so far this work has exhibited a lower response than PVDF. Recent work has included the use of zinc oxide (ZnO) wires as "nanopiezotronics:" combining semiconducting and piezoelectric properties for micropower generation, including hybrid fabrics generating up to 20 mW/m2.
These ZnO devices illustrate the promise of piezoelectric materials for energy harvesting, but conventional solid-state materials, such as ZnO, reflect a balance between piezoelectric response and conductivity. For example, high piezoelectric coefficients are found in PZT, which is a dielectric, while ZnO has higher conductivity and therefore potential for increased electrical generation, but a lower piezoelectric response. Consequently, we are investigating the use of designed molecular materials to simultaneously optimize both piezoelectric response and conductive properties.
We proposed to demonstrate single-molecule piezoelectric response, through high-level density functional theory (DFT) calculations and through experimental synthesis and characterization. We will develop rational structure/property relationships for high piezoelectric response and synthesize highly-active targets. We will also develop these materials into self-assembled multi-layer devices for energy harvesting.
In this first report, we will discuss our initial findings, including a methodology for computing piezoelectric deformation of single molecules, a study of substituents and regioisomers to produce structure/property relationships, identified an active target for synthesis, and initial characterization attempts.
Computational Study
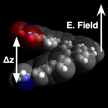
Figure 1. Orientation of molecule relative to applied electric field.
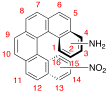
We have recently submitted our preliminary investigation into computational prediction of piezoelectric deformation of single molecules. Using the Gaussian computational chemistry package, we applied external electric fields with varying strengths along the long axis of helical molecules to force conformational changes, as illustrated in Figure 1. The optimized geometry as a function of applied field clearly illustrate the piezoelectric effect in Figure 2, for calculations on amino-, nitro- substituted [6]helicene molecules. For this particular compound, 4-amino-5-nitro[6]helicene (4a15n), the computed deformation of 12% corresponds to a piezoelectric coefficient (d33) of 45.8 pm/V, comparable to ZnO and much higher than PVDF.
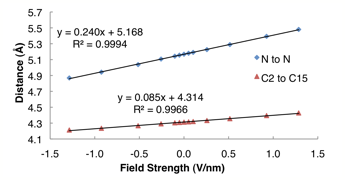
Figure 2. Calculated N to N and C2 to C15 distances in 4a15n under different applied external electric fields. Note that while the deformations are largely linear across the field strengths studied, some curvature does occur at high positive and negative applied fields.
We considered several other molecular systems, substituent effects, and different regioisomers of the helicenes to establish important correlations between molecular structure and piezoelectric deformation. Surprisingly, the dipole moment of the molecule has low correlation with the piezo response, even though the product of the applied field and dipole moment yield the potential energy difference that distorts the lowest-energy geometry. Instead, the degree of alignment of the molecular dipole moment with the applied field (not the magnitude) was most important.
Our computational studies have yielded structure/property relationships and demonstrated that the helicenes with high polarizability, reflect an ideal synthetic target.
Synthesis
We have begun synthesis of the bi-substituted helicenes. Most previous synthesis has been towards C2 symmetric helicenes, so both "top" and "bottom" substituents are identical. This particular synthetic route yields control of both functional groups and regiochemistry via a combination of C-C bond formation reactions and photoisomerizations.
We have prepared milligram quantities of the bromo-, methoxy
product and are now tailoring the procedure to produce new isomers and the
desired nitro functional group.
Characterization
In addition to computational studies over the last year and more recent synthetic efforts, we have pursued experimental verification of the single-molecule piezoelectric deformation effect using conducting-probe AFM measurements of self-assembled monolayers (SAMs). We have measured the deflection and force constant of piezoelectric molecules on the nanoscale. In the next year, we will modulate the force response with applied field, and correlate these nanoscale measurements with more conventional thin-film piezoelectric characterization techniques.
Summary of Activities
Over the initial period of the funding, we have provided clear structure/property relationships from our computational studies, demonstrating significant piezoelectric response for organic molecules using conformational changes under an applied electric field. We have synthesized bi-substituted helicenes, the target compounds shown to have high response from our computational studies, and are undertaking experimental characterization of the piezoelectric response in thin-films and monolayers.
Impact of PRF Award
The award for this research under the DNI program was the first outside research funding received by the Hutchison group. Consequently, it allowed our group to pursue a novel, risky research direction with little preliminary results. In response, we have significantly developed our initial computational results and submitted a manuscript to the Journal of Physical Chemistry Letters in the last month. The graduate student supported by this project has showed tremendous promise, completing the computational simulations, developing and executing the synthetic route to the bi-substituted [6]helicenes, and testing conducting-probe AFM and device characterization techniques. In short, he has developed into a complete, well-rounded researcher across computational, synthetic, and analytical domains.
We strongly believe that thanks to the ACS PRF support, this new research area will easily expand into novel energy harvesting applications and new areas of chemistry. By the end of the funding period, we will have multiple publications and substantial preliminary results to secure further funding from the NSF, DOE or other sources.
Copyright © American Chemical Society