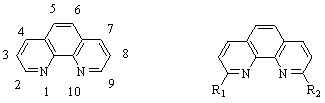AmericanChemicalSociety.com
Reports: B3 46743-B3: Homoleptic and Heteroleptic Copper(I) Phenanthrolines: Sterically Enhanced Excited States
Kurstan L. H. Cunningham, St. Norbert College
Background
This grant year allowed for the photochemical comparison of symmetric and asymmetric copper(I) complexes as represented by the ligands in Figure 1.
Figure 1: (Left) The numbering system for the 1,10-phenanthroline ligand.
(Right) General examples of symmetric (R1 = R2) and asymmetric (R1≠ R2) ligands.
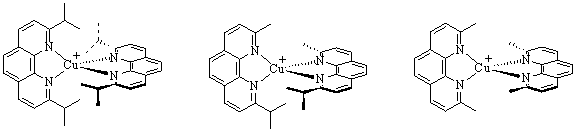
Asymmetric copper(I) phenanthroline complexes are not new to the field of coordination chemistry. In 1999, Sauvage et al. was the first to create a series of asymmetric phenyl derivatives of 1,10-phanthroline in an effort to understand the thermodynamic and kinetic properties of the copper(I) ion in an asymmetric environment.1 Photochemical studies were soon to follow when copper(I) complexes were synthesized using phenanthrolines with a variety of aryl groups attached at the 2 and 9 positions to create asymmetric ligands.2 The disadvantage to this approach was that these aromatic substituents can twist up to 60º from planar, decreasing their steric interaction with the pocket in which the copper(I) is contained and allowing for a low-energy deactivation of the excited state.3 Therefore, these aryl derivatives are not the ideal substituent for a photochemical investigation. The best investigation of this steric interaction of R groups influencing the photochemical properties of the complex should use a truly homologous series, which is the focus of this year's report. This project compares the steric interaction created by the symmetric 1,10-phenanthroline ligands having either methyl or isopropyl groups in the 2 and 9 positions with asymmetric ligand that contains both groups. Figure 2 shows the series of complexes successfully synthesized.
Cu(dipp)2+ Cu(ipmp)2+ Cu(dmp)2+
Figure 2: Structures of the three copper(I) complexes.
The left and right complexes are the symmetric complexes and the middle complex represents the asymmetric complex.
Synthesis
Symmetric ligands: The 2,9-dimethyl-1,10-phenanthroline (dmp) was purchased from Aldrich while the 2,9-diisopropyl-1,10-phenantholine (dipp) was synthesized as reported in the literature.4 The ligands were complexed by copper(I) according to an established procedure and the emission spectra was in accordance with the published data.4
Asymmetric ligand: The asymmetric ligand, 2-methyl-9-isopropyl-1,10-phenanthroline (ipmp) was initially prepared in a two-step procedure to add each R group in sequence. A published procedure was used to create the 2-methyl-1,10-phenanthroline.5 This intermediate compound was isolated and purified in a 70% yield before it was reacted with isopropyl lithium to create the novel 2-methyl-9-isopropyl-1,10-phenanthroline in a 50% yield (overall 35% yield). The 2-methyl-1,10-phenanthroline was also used to create the novel 2-t-butyl-9-methyl-1,10-phenanthroline; however, the copper complex of this ligand has not been purified by this reporting date.
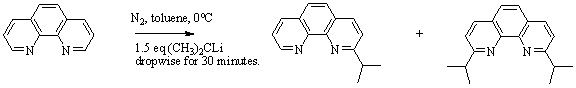
Considering our previous data reported in 2008-2009 to PRF about the reactivity of phenanthroline derivatives depending on the substituents on the ring, we decide to change the order of the R group's attachment to the parent ring and we noted surprising results. The new synthesis of 2-isopropyl-1,10-phenanthroline was accomplished by the following reaction in Figure 3.
Figure 3: Synthesis of the 2-isopropyl-1,10-phenanthroline was completed in an 80% yield. Byproduct of the reaction is the 2,9-diisopropyl-1,10-phenanthroline
which is also used in this study.
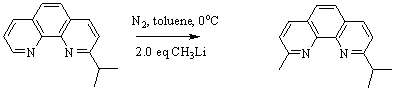
The isolated compound was then reacted with methyl lithium which converted 100% of the 2-isopropyl-1,10-phenanthroline into 2-methyl-9-isopropyl-1,10-phenanthroline (overall 80% yield) as seen in the following reaction in Figure 4. This new and improved synthesis increased the overall yield of the ligand by 45%.
Figure 4: Novel synthesis of the 2-methyl-9-isopropyl-1,10-phenanthroline.
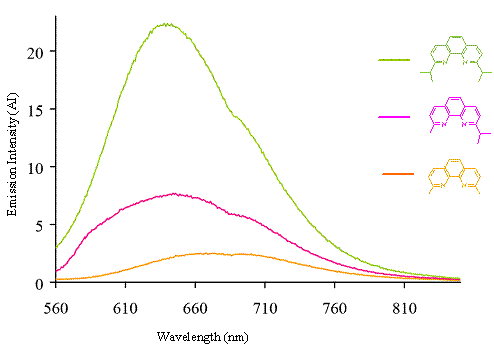
Copper complexes: The creation of the copper(I) complexes followed a published procedure to create the orange-red solids with the general formula of Cu(NN)2PF6 for each complex.4 These solids were dissolved in the non-coordinating solvent of dichloromethane to create solutions that were absorbance matched at 440 nm for the photochemical studies. Figure 5 is the overlay of the emission spectra for the three copper complexes.
Figure 5: Overlay of the three copper complexes.
As expected, the bis(2,9-diisopropyl-1,10-phenantholine)copper(I) complex (green line) has an emission wavelength and intensity indicative of a sterically locked copper complex compared to the smaller methyl group of the bis(2,9-dimethyl-1,10-phenanthroline)copper(I) complex (orange line). However, our data show the asymmetric copper complex (pink line) to have an emission maximum comparable to the symmetric isopropyl complex without gaining the corresponding increase in the emission intensity. We attribute this result to the asymmetry of the copper complex facilitating a non-radiative decay pathway to deactivate the excited state. Crystal structures of the solids are pending to confirm a possible distortion to support this claim.
The bis(2-t-butyl-9-methyl-1,10-phenanthroline)copper(I) complex is currently being purified away from unbound free ligand, which has an overlapping luminescence with the copper complex. The photochemistry of this complex is of interest not only as a companion piece to the above data, but also as an comparison to the previously reported heteroleptic 2,9-dimethyl-1,10-phenantholine)( 9-di-t-butyl-1,10-phenantholine)copper(I).6
This grant year involved my supervision of four undergraduates as a part of the PRF goal to involve students and “…contribute positively to educational and workforce development in scientific or technological field.”7 One student graduated in May and accepted an offer into the chemistry PhD program at Purdue University. Two others are seniors at SNC this year and are applying to chemistry graduate school, one of which is my SUMR scholar student researcher. This ongoing project was a topic of student presentations at Spring 2010 ACS meeting, one on-campus presentation and one regional presentation.
References:
1. Meyer, M; Albrecht-Gary, A-M., Dietrich-Buchecker, C.O.; Sauvage, J-P. Inorg. Chem., 1999, 2279-2287
2. a) Miller, M.T.; Gantzel, P.K.; Karpishin, T.B. Inorg. Chem. 1999, 3414-3422. b) Scmittel, M.; Michel, C.; Lui, S-L.; Schildbach, D.; Fenske, D. Eur. J. Inorg. Chem. 2001, 1155-1166.
3. Miller, M.T.; Gantzel, P.K.; Karpishin, T.B. Inorg.Chem. 1998, 2285-2290
4. Cunningham, K. L.H.; McMillin, D. R. Inorg. Chem. 1998, 37, 4114-4119.
5. Taylor, C.M.; Watton, S.P.; Flurie, A.;Ricketts, J.I. Inorg. Chem., 2003, 7381-7386.
6. Miller, M.T.; Gantzel, P.K.; Karpishin J. Am. Chem. Soc. 1999, 121, 4292-42933.
7. Undergraduate Research (UR) Grants (accessed 09/21/10)
Copyright © American Chemical Society