AmericanChemicalSociety.com
Reports: DNI4 48788-DNI4: Sources and Chemistry of Organic Aerosol Material
V. Faye McNeill, PhD, Columbia University
Glyoxal-Methylglyoxal Cross Reactions in Secondary Organic Aerosol Formation
Organic matter is a ubiquitous component of atmospheric aerosols. Inorganic aerosols may acquire an organic component via in situ interactions with volatile organic compounds (VOCs), a family of processes known as secondary organic aerosol (SOA) formation. SOA formation is one of the greatest sources of uncertainty in estimations of aerosol forcing on climate.
Glyoxal (G) and methylglyoxal (MG) are gas-phase oxidation products of many anthropogenic and biogenic volatile organic compounds (VOCs). In several cases, including isoprene and toluene oxidation, G and MG are both oxidation products of the same VOC. G and MG have recently attracted much attention as potential SOA precursors. SOA formation by these volatile a-dicarbonyl species occurs via their uptake into the aqueous phase of an aerosol particle or cloud droplet, followed by aqueous-phase chemistry leading to the formation of low-volatility oligomer products. While such heterogeneous SOA formation pathways have been identified for G and MG separately, these species are unlikely to be found in isolation in the atmosphere. These highly reactive species resemble each other structurally and participate in similar self-oligomerization chemistry in aqueous systems. Therefore, significant cross-reaction may occur when both G and MG are present. Cross-oligomerization between G and MG could increase the expected SOA yield from these precursors. Additionally, we have shown that the secondary organic products formed by G and MG in the presence of NH4+ and H3O+ may change the optical properties of the aerosol, and MG may affect the aerosol surface tension. In order to evaluate the atmospheric significance of these observations, we must study the SOA formation chemistry of G and MG as it would most likely occur in nature: simultaneously in a mixed system.
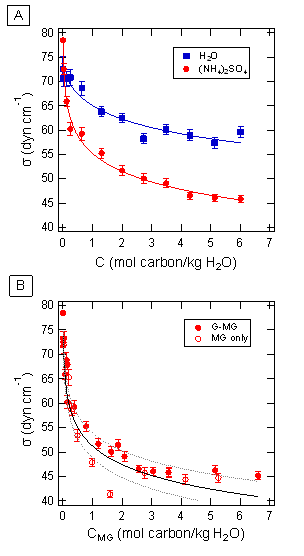
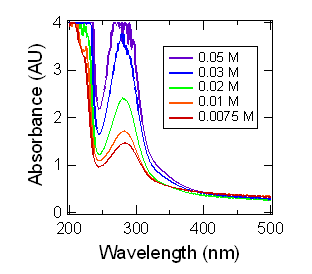
In this work, we studied the chemistry of G and MG together in aqueous aerosol mimics containing ammonium sulfate (AS). We characterized the formation of light-absorbing secondary organic products using UV-Vis spectrophotometry (Figure 1). We found that a model based on the formation kinetics and optical properties of self-reaction products is sufficient to describe the observed kinetics of formation of products absorbing at 280 nm. We also showed that the effects of G and MG on the surface tension of the solution are additive (Figure 2). Aerosol-CIMS was used to characterize reaction mixtures, and while most peaks observed corresponded to G or MG self-reaction products, some peaks consistent with products of G-MG cross-reaction by aldol condensation or hemiacetal formation mechanisms were observed. We concluded that G-MG cross-reactions contribute to SOA mass yield from these precursors, but that they need not be explicitly taken into account when modeling the effects of G and MG uptake on aerosol surface tension or light absorption.
Methylglyoxal Uptake Enhances Aerosol CCN Activity
Atmospheric aerosol particles nucleate cloud droplets, thereby influencing the radiative properties, amount and lifetime of clouds, also known as the aerosol “indirect effects” on climate. The relationship between an aerosol particle's chemical composition and its ability to nucleate cloud droplets (CCN ability) is complex. Organic aerosol material (OA), a common component of tropospheric aerosols, is typically more hydrophobic, and therefore less hygroscopic than inorganic salts. Surface-active OM can lower aerosol surface tension, thus enhancing CCN activation however, an organic surface film can also act as a kinetic barrier for water uptake to the aerosol. The reactive uptake of the trace gas methylglyoxal by aqueous particles and cloud droplets is a potential source of OA; methylglyoxal is known to form oligomers and depress surface tension in bulk aqueous solutions. Here we show that ammonium sulfate particles exposed to gas-phase methylglyoxal in an aerosol reaction chamber activate at sizes up to 15% lower at a given supersaturation than do pure ammonium sulfate particles. Enhanced CCN activity of small particles may lead to increased cloudiness, smaller cloud droplets, and decreased precipitation. This is the first direct evidence that volatile organic compounds may enhance aerosol CCN activation.
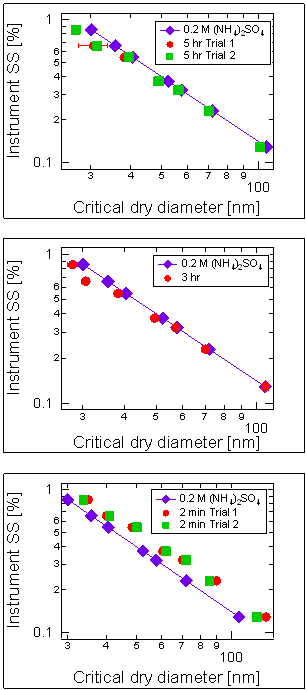
We studied the changes in the CCN activity of ammonium sulfate seed aerosols upon exposure to gas-phase methylglyoxal. A cloud condensation nuclei counter (CCNC) was used downstream of a 3.5 m3 Teflon reaction chamber or an aerosol flow tube in order to determine the cloud-forming potential of these aerosols after various exposure times. We find that on a timescale of hours, methylglyoxal exposure enhances CCN activation at the conditions studied (Figure 3). To our knowledge this is the first direct experimental evidence that the uptake of gas-phase organic precursors to atmospheric aerosol particles may lead to an enhancement in cloud droplet nucleation. These results further assert the importance of organics in determining the CCN activity of atmospheric aerosol particles. This study introduces the idea that volatile organics in the atmosphere may act as a reservoir of surfactants that can be taken up by aerosol particles, thereby affecting CCN activity.
Figure 1. UV-Vis spectra of aqueous
solutions containing 3.1 M (NH4)2SO4 and
varying total organic concentration (G:MG 1:1). Spectra were measured 24 h
after mixing. Figure 2. Pendant drop tensiometry
results. Surface tension is shown for A) aqueous solutions with and without 3.1
M (NH4)2SO4, with G:MG 1:1 at various initial
concentrations, and B) for 3.1 M (NH4)2SO4 and
a total organic concentration of 0-2 M, with varying ratios of G:MG, plotted as
a function of MG concentration. The curves in panel (A) are weighted nonlinear
least squares fits to the data using the Szyszkowski-Langmuir equation (eq. 7).
MG-only data from Sareen et al. (2010) along with the Szyszkowski-Langmuir
curve and confidence intervals from fits to that data are shown for reference
in panel (B). Each point represents the weighted average of five to eight
measurements, taken at 24 hours after mixing, and the error bars indicate the
standard deviation.
Figure 3: CCNC data from a chamber
experiment involving methylglyoxal and (NH4)2SO4
seed aerosols, for different exposure times.
Copyright © American Chemical Society