AmericanChemicalSociety.com
Reports: DNI10 48882-DNI10: Layered Rare-Earth Antimony and Bismuth Suboxides as Novel Thermoelectric Materials
Yurij Mozharivskyj, McMaster University
Objective:
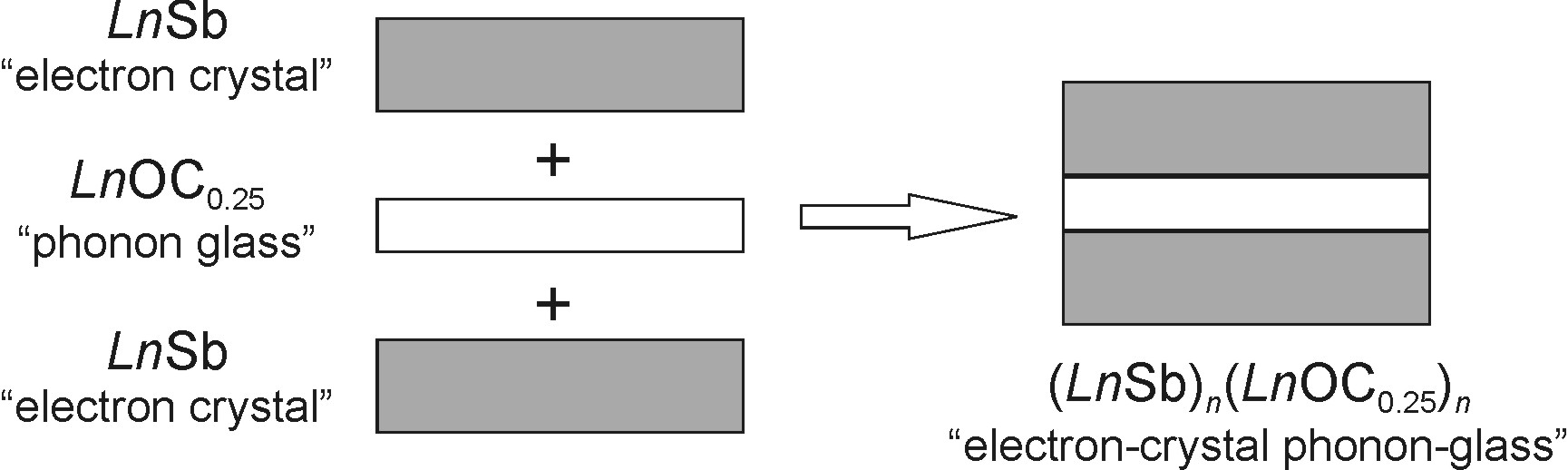
The goal of the ACS-PRF funded project was to prepare novel thermoelectric materials via chemical fusion of semimetallic RESb/REBi and insulating RE2O3 binaries (RE is a rare-earth element). The proposed research also focused on new layered oxycarbides with a general formula (RESb)n(REOC0.25) or (REBi)n(REOC0.25). The idea was to structurally combine the LnSb/LnBi and LnOC0.25 phases with different properties in order to obtain "electron-crystal phonon-glass" materials with improved thermoelectric performance (Figure 1).
Figure 1. Formation of (LnSb)n(LnOC0.25) from LnSb, an "electron crystal", and LnOC0.25, a "phonon glass".
Results:
1) RE3SbO3 and RE8Sb3-d O8 antimonide oxides.
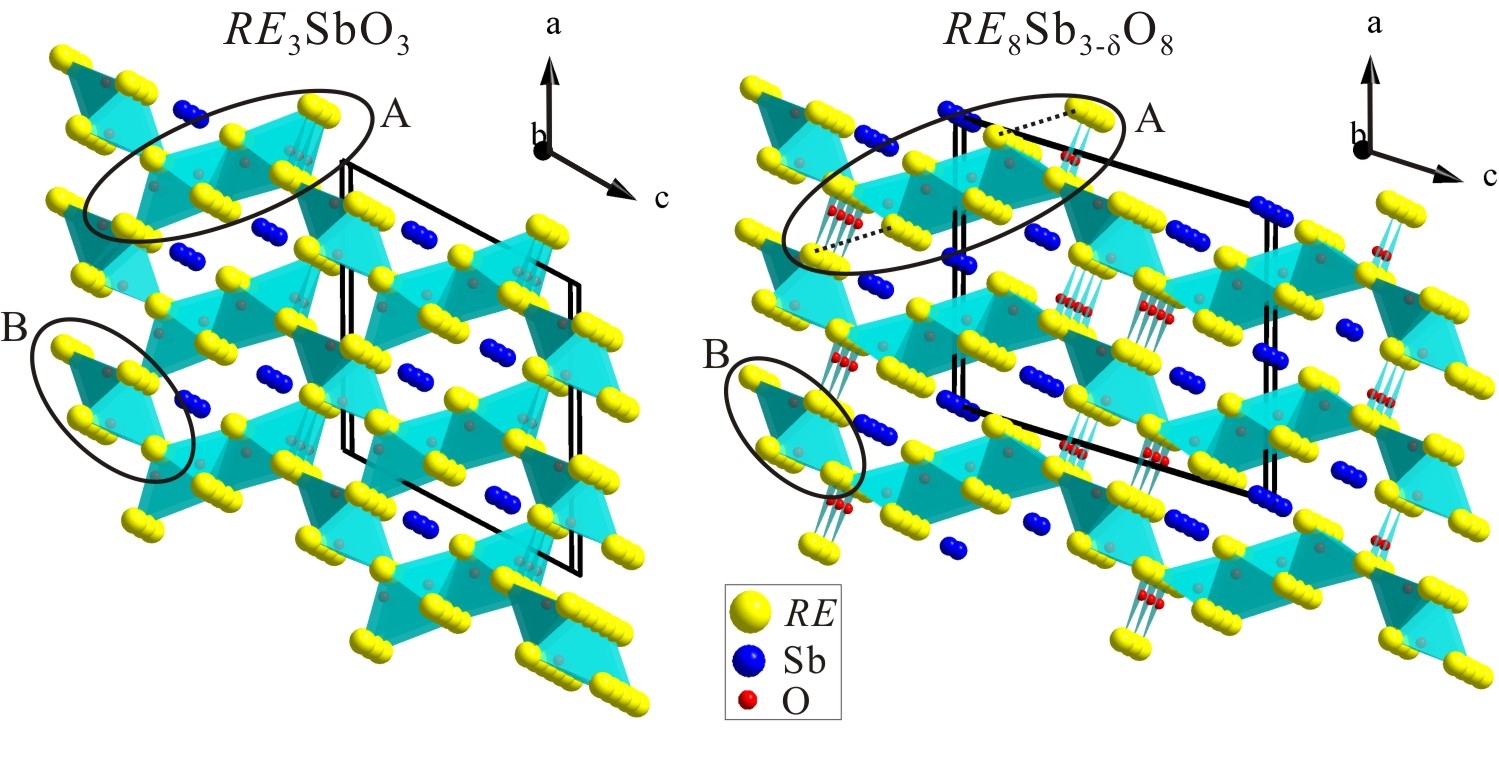
In the search of high-temperature thermoelectric materials, two families of novel narrow band-gap semiconducting suboxides with the RE3SbO3 and RE8Sb3-d O8 compositions (RE = La, Sm, Gd, Ho) have been discovered (Figure 2). Their synthesis was motivated by attempts to open a band gap in the semimetallic RESb binaries through a chemical fusion of RESb and corresponding insulating RE2O3. Temperatures of 1350oC or higher are required to obtain these phases. Both RE3SbO3 and RE8Sb3-d O8 adopt new monoclinic structures with the C2/m space group and feature similar REO frameworks composed of "RE4O" tetrahedral units. In both structures, the Sb atoms occupy the empty channels within the REO sublattice. High-purity bulk Sm and Ho samples were prepared and subjected to electrical resistivity measurements. Both the RE3SbO3 and RE8Sb3-d O8 (RE = Sm, Ho) phases exhibit a semiconductor-type electric behavior. While a small band gap in RE3SbO3 results from the separation of the valence and conduction bands, a band gap in RE8Sb3-d O8 appears to result from the Anderson localization of electrons.
Discovery of RE3SbO3 and RE8Sb3-d O8 opens a new chapter in the chemistry of rare-earth suboxides, which was barely explored until now.
Figure 2. Structure of RE3SbO3 and RE8Sb3-d
O3
2) Layred (RESb)n(REOC0.25) antimonide oxycarbides
We were also able to produce layered oxycarbides (HoSb)1.25(HoOC0.25) and (DySb)2.5(DyOC0.25). Their structures consist of two and four layers of HoSb and DySb and one layer of HoOC0.25 and DyOC0.25, respectively, stacked along the z direction (Figure 3). To our knowledge, such composite structures are unique. No physical properties were measured as only small single crystals were obtained.
These results provide a solid evidence for the existence of novel layered suboxides with a general formula (LnPn)n(LnOC0.25).
Figure 3. Structures of (DySb)2.5(DyOC0.25) and (HoSb)1.25(HoOC0.25).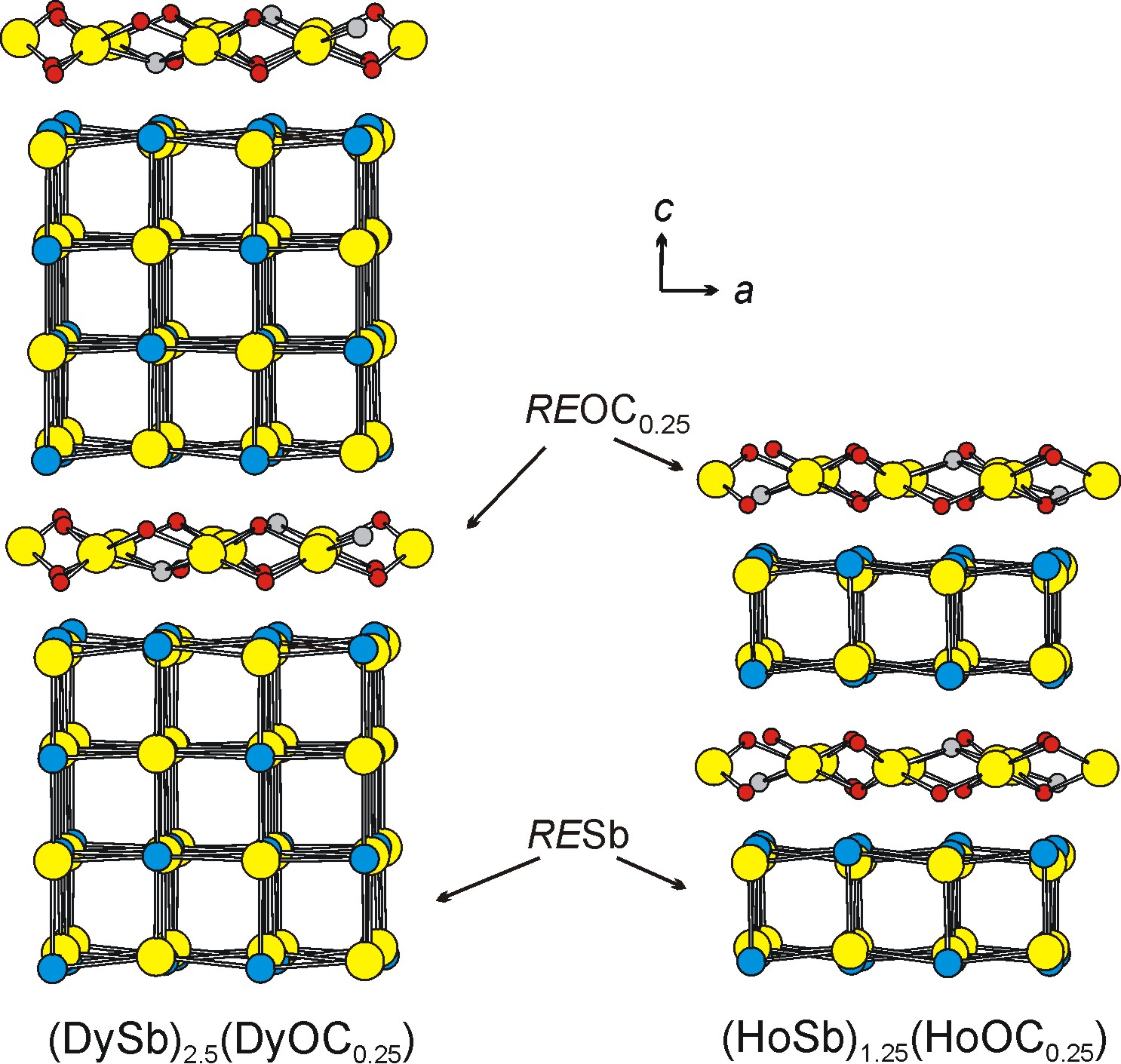
Copyright © American Chemical Society