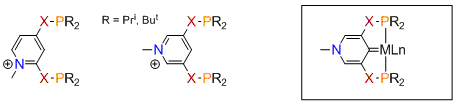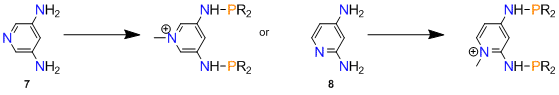AmericanChemicalSociety.com
Reports: AC3 47729-AC3: Abnormal Pincer-Type Carbenes for Functionalization of Hydrocarbons
Dmitri G. Goussev, Wilfrid Laurier University
The applications of pincer metal complexes in homogeneous catalysis and organometallic synthesis is an area of active chemical research, particularly in reactions where functionalization of hydrocarbons is desirable through C-H bond activation. We are developing novel pincer ligands possessing a central pyridylidene fragment. These can be derived from 2,4- and 3,5-substituted pyridinium ions shown in Scheme 1 where the phosphorus groups are attached to the central ring via O, NH, or CH2 groups. A generalized structural example of a 3,5-pyridylidene pincer complex is shown below.
Scheme 1.
In year 1, we investigated synthetic routes toward the phosphinite compound [2,4-(OPBut2)2-C5H3NMe]+ (Scheme 1, left: X = O, R = But). In this case, when X = O, there is no trivial synthetic approach to selective N-alkylation during the ligand synthesis. This problem was circumvented by introduction of a methyl substituent at C-6 that provided steric protection for the pyridine nitrogen against metal coordination. This way we were able to synthesized ruthenium and iridium pincer complex precursors that were subsequently selectively N-alkylated to give first examples of 2,4-pyridylidene metal compounds.
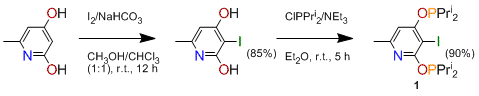
In year 2, this work was extended in several ways. We first sought (a) to reduce the size of groups on phosphorus, expecting more active catalysts to have less steric congestion around the central metal, and (b) to introduce a halogen atom at C-3 to facilitate formation of the metal-carbon bond. The targeted system (1) was obtained in high yield according to Scheme 2:
Scheme 2.
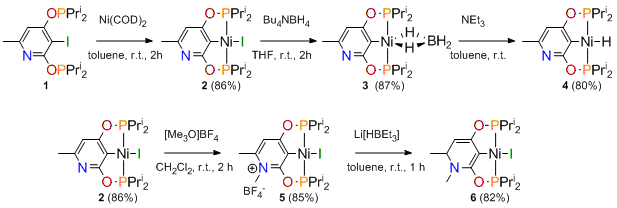
We further investigated the reactivity of 1 with several metal precursors. A series of nickel complexes were successfully derived from 1 and Ni(COD)2. The nickel chemistry is summarized in Scheme 3:
Scheme 3.
Reactions of 1 with [RuCl2(p-cymene)]2, RuCl2(PPh3)3, RuCl3∙3H2O, IrCl3∙3H2O, [IrCl(COE)2]2, [IrCl(COD)]2 were not productive and afforded intractable mixtures. Based on NMR studies, it appeared that the main problem with 1 (as well as the related phosphinites prepared in year 1) might be hydrolytic instability. We have found that such phosphinites generally poorly tolerate protic solvents (e.g., methanol, ethanol, 2-propanol) which are often indispensible in the syntheses of organometallic compounds. Therefore, we decided to explore the synthesis of pyridylidene pincer ligands containing phosphorus groups linked with the central pyridine ring via X = NH, rather than X = O, groups.
Scheme 4
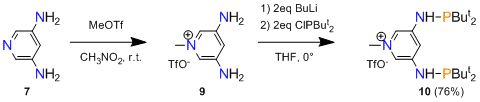
3,5- and 2,4-diaminopyridines 7, 8 are natural starting materials for the desired aminophosphines of Scheme 4. However, unlike pyridinediols, such diaminopyridines are not commercially available. An added problem was that the reported literature yields were unsatisfactory for 7 and 8. When we tried a literature method of making 7 by heating 3,5-dibromopyridine in aqueous ammonia in the presence of a Cu(II) catalyst, we found that the product could not be easily recovered from the reaction mixture. We therefore decided to invest time in the development of an efficient and productive synthesis of 7, since it was clear that without readily available diaminopyridines further work toward the ligands in Scheme 4 would not be practical. Gratifyingly, a high-yield synthesis of sufficient quantities of 7 (4 g, 86%) was achieved by heating a solution of 3,5-dibromopyridine in a 7N solution of NH3 in methanol, in the presence of 4 mol% copper(I) oxide catalyst, at 130 °C. The product conveniently crystallized from a methanol/toluene mixture upon evaporation. Remaining steps in the synthesis of the 3,5-bis(aminophosphine)pyridinium salt 10 (Scheme 5) presented no problems. Pyridine N-alkylation was fast and selective and afforded the pyridinium salt 9 quantitatively. Aminophosphine 10 proved to be stable toward water and could be conveniently washed with H2O during purification.
Scheme 5.
Through the syntheses of 7 and 9 we have now obtained a sufficient (ca. 7 g) quantity of 10 adequate for a large-scale exploration of the reactivity of this ligand with transition metals from groups 9 and 10. Recent experiments with 10 and [RuCl2(p-cymene)]2 revealed a clean formation of a pyridylidene monohydride complex formulated according Scheme 6 based on NMR data.
Scheme 6.
Continuing work on this project is planned toward the following targets: (a) synthesis of osmium and iridium complexes related to 11 and investigation of their structure, reactivity, and catalytic properties, (b) synthesis of an analogue of 10 with –PPri2 groups and investigation of its metal chemistry. Future work should also involve synthesis of 8 (following our successful method for 7) and use of 8 to make 2,4-bis(aminophosphine)pyridinium salts.
Copyright © American Chemical Society