AmericanChemicalSociety.com
Reports: ND5 49192-ND5: Development Of A Scattering Characterization Technique To Study The Nucleation And Growth Mechanism Of Supported Metals
Maria Martinez-Inesta, PhD, University of Puerto Rico
Background:
This New Directions Project officially started in June 2009. During this year the PhD student Liliana Gámez was recruited to work in it as her dissertation work. The objectives of this project were to develop the X-ray Characterization technique pair distribution function as a probe to study the nucleation and growth mechanisms of supported catalysts. The results described below obtained thanks to this grant have been presented in posters, are being developed into a first publication and have allowed the PI to submit a related proposal to NSF as funding to further the experiments and analysis proposed in this grant. In addition the PI has incorporated part of the knowledge acquired while working in this grant in her graduate elective course X-ray Characterization of Materials last semester.
Results:
Initial experiments on the supported catalysts were done using zeolite X as a support for platinum catalysts. The preparation and dispersion of the supported metal samples consisted of 3 phases: Incorporation of the metal catalyst into of the support through the ion exchange (I.E.) method described by deGraaf and dry impregnation (D.I.) using platinum ammonium nitrate as the Pt precursor, drying and oxidation at different heating rates and reduction of the metal.
In house X-ray Diffraction Characterization
The zeolite support and the Pt loaded samples were studied first in situ in an in house with X-ray diffractometer using an attachment called reactor X by the Rigaku Corporation. This attachment allows the flow of reactive gases at high temperatures through a sample while simultaneously acquiring their X-ray diffraction patterns (XRD's), through which in situ crystallographic data of the structural changes of the catalysts during nucleation and growth can be obtained.
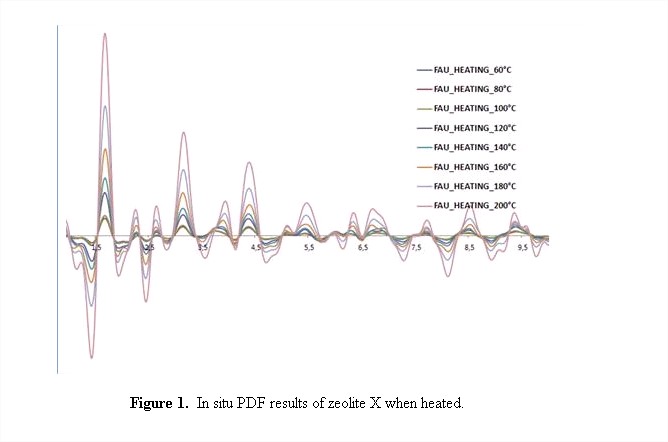
Initial XRD results obtained in situ for zeolite X at different temperatures under an oxygen and nitrogen atmosphere evidenced that this zeolite undergoes significant structural changes during heating due to its hydrophilicity. As a consequence it is evident that in order to obtain reliable information on the structural changes related to the platinum a comparison must be made with the XRD pattern of the zeolite at the corresponding temperature.
No structural change was observed during the oxidation phase at 0.5 and 1°C/min. This does not mean that there are no changes occurring during oxidation but probably that the changes are too small to be observed with this technique. During reduction a platinum <111> peak was observed in all samples.
Pair Distribution Function Experiments
Based on the in house XRD results several catalysts were synthesized ex situ to be characterized using high energy X-rays at the 11-ID-B beamline at the Advanced Photon Source (APS) at Argonne National Laboratory, Argonne, Illinois (USA) with our pair distribution function experiments on March 2010 and two were studied in situ, zeolite X under oxygen and an ion exchanged 1.5% wt Pt/zeolite during oxidation and reduction. The ex situ samples studied included the untreated samples with different Pt loadings, the samples oxidized at 1°C/min and 0.2°C/min and the samples oxidized and reduced at 1°C/min and 0.2°C/min.
X-rays with a 0.2128 Å wavelength and a polarization factor of 0.95 were used for the experiments. Scattered radiation was collected with an Qmax of 25 Å and the data were normalized for flux, corrected for detector dead time, background scattering, absorption, multiple scattering, polarization, Laue diffuse scattering, Compton scattering and reduced to the total structure factor S(Q), using the program PDFgetX2 to obtain the pair distribution function (PDF) of the samples.
The PDFs of the zeolite X studied in situ are shown in Figure 1.Significant structural changes are observed at all interatomic distances. Contribution to this changes are both from desorption of water and temperature. We are currently analyzing these results crystallographically to propose models for these structural changes.
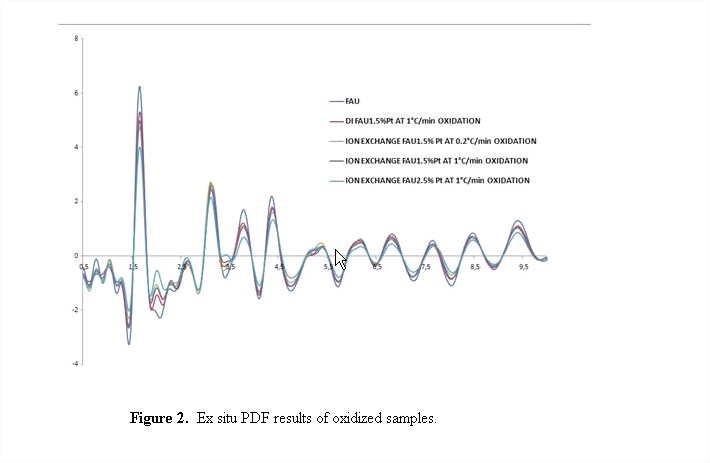
All the untreated samples show the same peaks with similar intensities. After oxidation, however, changes in the intensities of the peaks can be observed up to 5 Å as shown in Figure 2. This is in contrast to the results observed in the in house oxidation XRD experiments. This evidences that this X-ray technique is more sensitive to the structural changes of the metal precursor during nucleation and growth than regular XRD. A detailed differential PDF analysis to understand these structural changes is underway.
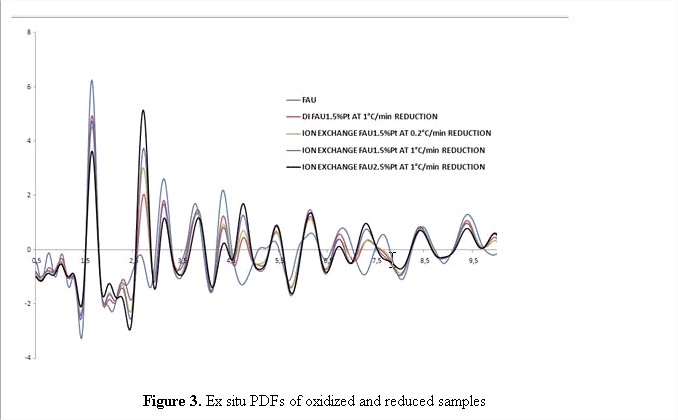
It is after reduction that the most significant differences appear. All the samples showed new peaks at approximately 2.70 Å and 4.75 Å, which suggest an FCC configuration of the platinum crystals inside the support. The relative height and width of these two peaks in all the samples suggest different dispersion and sizes in which surprisingly the DI 1.5% Pt/zeolite sample had the best results and the IE 2.5% Pt/X sample appear to have the largest clusters. In the near future we will be characterizing these samples using transmission electron microscopy. This will allow us to correlate the PDF results with the actual sizes and polydispersities of the Pt clusters in the samples. This is necessary to develop better structural models during their nucleation and growth which is our ultimate goal. In order to help us develop a complete nucleation and growth model we are also attempting Pt Solid State NMR in order to determine the feasibility of the technique to determine the Pt location in the untreated samples.
On our last visit to the Argonne National Laboratory facilities, on August 3,2010, an additional 70 ex situ PDF experiments were done using zeolite X, zeolite mordenite (Si/Al=6.5) and zeolite ZSM-5 (Si/Al=140) as supports. On this visit we also studied samples prepared with higher Pt loading, samples loaded with Pd and Ni and bimetallic samples. Due to the volume and complexity of the analysis, these results are still under study.
Copyright © American Chemical Society