AmericanChemicalSociety.com
Reports: AC7 44247-AC7: Complementary Hydrogen Bonding in Highly Branched Macromolecules: Thermoreversible Supramolecular Architecture for Improved Melt Processibility and Performance
Timothy E. Long, Virginia Polytechnic Institute and State University
Complementary
multiple hydrogen bonding polymers offer potentially superior mechanical integrity
compared to conventional hydrogen bonding polymer systems. Moreover, CMHB units provide an
efficient molecular recognition and self-assembly process due to the high
hydrogen bonding association constant. Nucleobase-containing linear and branched copolymers
exhibited DNA-like melting behavior and a stronger temperature dependence of
melt viscosity compared to non-hydrogen-bond containing polymers, suggesting
possible advantages as thermo-responsive polymers to self-heal at relatively
low temperatures. Utilizing
CMHB units in the design of a self-healing polymer that is both reusable and
durable may facilitate recycling of hard-to-dispose plastics for environmental
security and energy saving. Our studies start with determining the
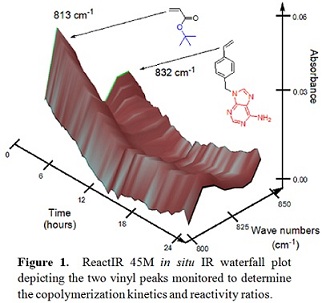
reactivity ratios of styrenic adenine monomer (VBA) and tert-butyl acrylate (tBA)
utilizing our newest instrument, a Mettler-Toledo ReactIR 45M, which allows us
to monitor in situ the infrared
spectrum of the reaction contents and ultimately the reaction kinetics. (Figure 1) According to the Mayo-Lewis
equation, the reactivity ratios for styrenic adenine and tBA were determined to be 0.41±0.02 and 0.42±0.02, respectively.
The literature reactivity ratios for styrene and tBA were 0.89 and 0.29, respectively, in toluene. The difference in
the reactivity ratios resulted from the change of solvent, which led to a
higher Collaborations with Eastman Chemical and KratonTM
Polymers revealed the suitability of this novel functional hydrocarbon platform
for both adhesive and elastomer technologies, respectively. Studies showed that CMHB greatly influenced
corresponding polymer physical properties, such as mechanical and morphological
behavior. Our attention on tailored, self-assembled nucleobase-containing
polymers as membranes provides structure and ionic transport relationships that
suggest block copolymer structures for desalination and ion-transport. Current efforts are focused on
the preparation of adenine and thymine based on acrylate functionality. The
monomers will be used to demonstrate the role of sequenced hydrogen bonding on
morphological and rheological performance.
Moreover, our efforts will continue to address the new area of
"supramolecular click" chemistry wherein the functional group is located on the
polymer template using molecular recognition. An important question arises
concerning the selective nature of the functionalization, and the ability for a
molecular guest to selectively locate the complementary pair in the presence of
other nucleobases is an important aspect of continued research efforts. ADDIN
EN.REFLIST
 Nature's ability to precisely assemble
macromolecules into highly cooperative and functional assemblies, such as DNA,
provides an inspiration for our proposed efforts. Our research program involves
novel synthetic strategy to tailor the relationship between macromolecular
structure and non-covalent interactions for the discovery of novel functional,
hydrogen bonding macromolecules. Our
current research efforts involve the discovery of nucleobase-containing
hydrogen bonding polymers with tunable mechanical properties for melt
processing and self-healing applications. The primary principle is the ability
to control the mobility and accessibility of the associative site, and
disassociation in the melt step will enable more facile processing of
thermoplastic elastomers containing ionic sites. Complementary multiple hydrogen bonding (CMHB) has received relatively
sparse attention as a site for molecular recognition and intermolecular
interactions. The foundation for this program is
based on free radical copolymerization for the preparation of
nucleobase-containing polyelectrolytes and polyacrylates.
Nature's ability to precisely assemble
macromolecules into highly cooperative and functional assemblies, such as DNA,
provides an inspiration for our proposed efforts. Our research program involves
novel synthetic strategy to tailor the relationship between macromolecular
structure and non-covalent interactions for the discovery of novel functional,
hydrogen bonding macromolecules. Our
current research efforts involve the discovery of nucleobase-containing
hydrogen bonding polymers with tunable mechanical properties for melt
processing and self-healing applications. The primary principle is the ability
to control the mobility and accessibility of the associative site, and
disassociation in the melt step will enable more facile processing of
thermoplastic elastomers containing ionic sites. Complementary multiple hydrogen bonding (CMHB) has received relatively
sparse attention as a site for molecular recognition and intermolecular
interactions. The foundation for this program is
based on free radical copolymerization for the preparation of
nucleobase-containing polyelectrolytes and polyacrylates.  incorporation of tBA
than expected. Subsequent deprotection of tBA yielded water dispersible
nucleobase-containg polyanions, which mimic DNA structures. Nucleobase-containing
polycation analogs were salt-responsive in NaCl aqueous solution. Incorporating
CMHB sites in polyelectrolytes provide the potential for rheology modifiers to
assist nanofiber formation in the electrospinning process. (Figure 2) Additionally, the
intermolecular interaction of the complementary hydrogen bond and pi-pi
stacking interaction possibly plays a similar kinetic role as in nylon-6 in
regulating the chain orientation process and allowing tunable fiber rigidity.
Future studies will also address the salt responsiveness of nanofibers for
water purification application.
incorporation of tBA
than expected. Subsequent deprotection of tBA yielded water dispersible
nucleobase-containg polyanions, which mimic DNA structures. Nucleobase-containing
polycation analogs were salt-responsive in NaCl aqueous solution. Incorporating
CMHB sites in polyelectrolytes provide the potential for rheology modifiers to
assist nanofiber formation in the electrospinning process. (Figure 2) Additionally, the
intermolecular interaction of the complementary hydrogen bond and pi-pi
stacking interaction possibly plays a similar kinetic role as in nylon-6 in
regulating the chain orientation process and allowing tunable fiber rigidity.
Future studies will also address the salt responsiveness of nanofibers for
water purification application. 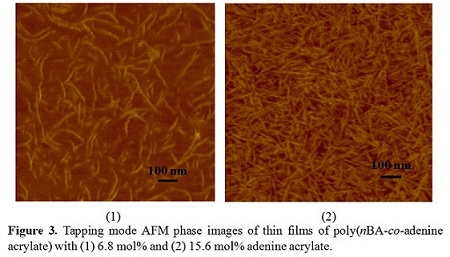 Our previous studies on styrenic
adenine- and thymine-containing polyacrylates showed that the bulky styrene
units connected to nucleobase functionality reduced polymer backbone mobility
and nucleobase binding efficiency in polymer blends. This observation catalyzed our current research program on the
original preparation of a novel acrylate nucleobase monomer containing a
dangling nucleobase (adenine and thymine) to improve binding and demonstrate
the CMHB influence on polymer adhesive properties. We have synthesized a series of acrylate adenine-containing random
copolymers. Figure 3 shows that
adenine units microphase separated from the amorphous low Tg polymer matrices and formed
percolated nano structures with a diameter of around 20 nm. This microphase
separation might be driven by pi-pi stacking interactions between adenine
units, which then contribute to self-healing as well as mechanical integrity.
The strength and reversibility of non-covalent interactions are highly
dependent on environmental conditions, and dynamic molecular recognition
processes for self-healing have not been significantly addressed in the
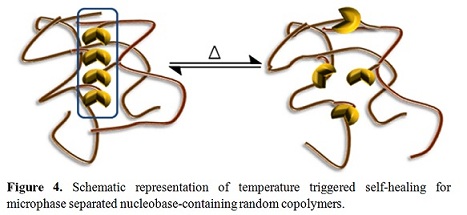
literature. Figure 4
depicts the building blocks for the introduction of pendant nucleobases and the
implications of tailored hydrogen bonding on self-healing.
Our previous studies on styrenic
adenine- and thymine-containing polyacrylates showed that the bulky styrene
units connected to nucleobase functionality reduced polymer backbone mobility
and nucleobase binding efficiency in polymer blends. This observation catalyzed our current research program on the
original preparation of a novel acrylate nucleobase monomer containing a
dangling nucleobase (adenine and thymine) to improve binding and demonstrate
the CMHB influence on polymer adhesive properties. We have synthesized a series of acrylate adenine-containing random
copolymers. Figure 3 shows that
adenine units microphase separated from the amorphous low Tg polymer matrices and formed
percolated nano structures with a diameter of around 20 nm. This microphase
separation might be driven by pi-pi stacking interactions between adenine
units, which then contribute to self-healing as well as mechanical integrity.
The strength and reversibility of non-covalent interactions are highly
dependent on environmental conditions, and dynamic molecular recognition
processes for self-healing have not been significantly addressed in the
literature. Figure 4
depicts the building blocks for the introduction of pendant nucleobases and the
implications of tailored hydrogen bonding on self-healing.
Copyright © American Chemical Society