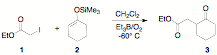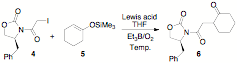AmericanChemicalSociety.com
Reports: UNI1 49489-UNI1: Stereoselective Tin-Free Radical Reactions
Jake R. Zimmerman, PhD, Ohio Northern University
My research group has benefited tremendously from the support of the ACS-PRF-UNI grant that we received. It has allowed me to fund three summer research students over the past two summers and purchase a variety of expendables that were needed for this project. Two of the students are currently beginning their senior year at ONU and both plan to attend graduate school in chemistry. Because of this support, my group has accumulated a lot of exciting results. I am very close to submitting two manuscripts for publication which will acknowledge ACS-PRF-UNI for its support. An undergraduate research student and myself presented two posters at the spring ACS National meeting in San Francisco, CA in March 2010 (abstract numbers ORGN 171 and ORGN 161). Below is a summary of my group's progress over the past fifteen months (June 1, 2009 through August 31, 2010).
In our initial studies on this
project, we wanted to develop an optimized protocol for electrophilic radical
additions onto electron rich alkenes at low temperatures (i.e. silylenol
ethers). Table 1 shows a summary of the results for this radical
fragmentation reaction using iodoethyl acetate (1) as the
electrophilic radical precursor and 1-cyclohexenyloxy timethylsiloxane (2) as the acceptor. We were pleased to find that this
reaction proceeds at low temperature and without solvent (see entry 4). A brief study on radical precursors was also conducted
and it was found that a-iodo esters, amides and ketones all give the target molecule in good
yields. It was observed, however, that a-bromo precursors are very inefficient in this
reaction (see entry 7, Table 1). Table 1. Low temperature radical fragmentation
reaction.
Entry R X Equiv 1 Equiv 2 Yield %a 1 -OEt I 1 1 68 2 -OEt I 1 2 75 3 -OEt I 2 1 85 4 -OEt I 4 1 81b 5 -OtBu I 2 1 60 6 -NH2 I 2 1 71 7 -Ph Br 2 1 12 a
Isolated yield. b Reaction was run without solvent. We
next turned our attention to a diastereoselective method using chiral
auxiliaries on the radical precursor. The reactions proceeded with moderate to
good yields both at room temperature and -60 ûC. The products, however, showed
little to no diastereoselectivity using the traditional Evan's chiral auxiliary
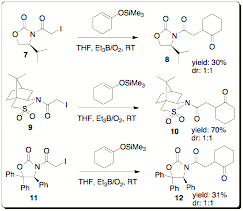
(see Table 2). A more extensive auxiliary screening gave similar results with
almost no dr observed (Scheme 2). We plan to continue this study broadening the
chiral auxiliary screening. We have also begun investigating an
enantioselective variant of this reaction. Only minimal amounts of
stereocontrol are present in our initial attempts on this asymmetric reaction.
Therefore, we propose to carry out an exhaustive CLA catalyst screening for
this asymmetric radical process. Table 2. Diastereoselective
radical fragmentation reactions.
Entry Lewis acid (0.5 equiv) Temp. (°C) dra Yield %b 1 -- 25 1:1 72 2 Mg(ClO4)2 25 1:1 62 3 -- -60 1:1 47 4 Mg(ClO4)2 -60 1:1 28 5 In(OAc)3 -60 1:1 75 aDetermined
by 1H NMR (200 MHz) b Isolated yield. Scheme 2. Chiral auxiliary
screening. Our
group is also investigating a tin-free radical project that utilizes simple
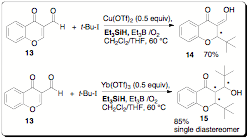
silanes as hydrogen atom sources. In this study we screened a variety of
silanes and Lewis acids in the radical additions onto 3-formylchromone (13), a commercially available starting material (Scheme
3). Interestingly we found that weaker Lewis acids such as Cu(OTf)2
give product 14 after a one hour
reaction time. If a stronger Lewis acid such as Yb(OTf)3 is
utilized, then the double addition product 15 is isolated as a single diastereomer. Even more
interesting is the fact that triethylsilane serves as an efficient hydrogen
atom source in this low temperature radical reaction. We completed a survey of
silane reagents and found that triethylsilane was optimal in this reaction. We
are currently investigating the asymmetric version of this reaction using a
variety of chiral Lewis acid catalysts. Scheme
3. Silane mediated radical additions. References
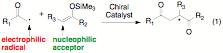 The most commonly reported
asymmetric free radical reaction involves an alkyl radical addition onto an
enone or imine which makes the overall scope of products very limited.1
Therefore, our goal was to develop a new asymmetric free radical method by
switching the usual mode of reactivity by adding electrophilic radicals,
generated from a-iodo carbonyl compounds, onto
electron rich enol ethers (scheme 1). The products obtained from these
reactions are 1,4-dicarbonyls which could be useful small molecule building
blocks. By using a chiral catalyst, high levels of stereocontrol could be
achieved. Since this reaction proceeds through a radical fragmentation pathway wherein
a TMS radical propagates the chain, there is no need for the use of toxic
alkyltin2 reagents in this process.
The most commonly reported
asymmetric free radical reaction involves an alkyl radical addition onto an
enone or imine which makes the overall scope of products very limited.1
Therefore, our goal was to develop a new asymmetric free radical method by
switching the usual mode of reactivity by adding electrophilic radicals,
generated from a-iodo carbonyl compounds, onto
electron rich enol ethers (scheme 1). The products obtained from these
reactions are 1,4-dicarbonyls which could be useful small molecule building
blocks. By using a chiral catalyst, high levels of stereocontrol could be
achieved. Since this reaction proceeds through a radical fragmentation pathway wherein
a TMS radical propagates the chain, there is no need for the use of toxic
alkyltin2 reagents in this process.
Copyright © American Chemical Society