AmericanChemicalSociety.com
Reports: DNI10 49045-DNI10: Metal Oxide Nanoparticles for High Energy Electrochemical Capacitors
Gleb Yushin, PhD, Georgia Institute of Technology
Two types of carbon substrate electrodes were investigated during the course of this study. One electrode consisted of acetylene black powder (95 wt. %) bonded with a polytetrafluoroethylene (PTFE) binder (5 wt. %). The second electrode was composed of carbon nanotubes with no PTFE. The CNT utilized were first grown in our laboratory, then boiled in a mixture of nitric and sulfuric acid in a 1:1 ratio for 1 hour at 100°C, sonicated and deposited on a filter to produce a thin porous film. The acid purification step was done in order to introduce defects and surface functionalities onto the surface of the carbon nanotubes. These defects serve as nucleation cites for the subsequent deposition of transition metal nanoparticles or thin films from the vapor phase. In addition, these defects may increase the rate of electron transfer from carbon to metal oxide.
Thin films of metal oxides have been deposited on carbon electrodes from the vapor phase. X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM), and Energy-Dispersive X-Ray Spectroscopy (EDS) were used to characterize the microstructure and identify the growth products on the carbon substrates. XRD measurements were made on a X-Pert Pro Alpha-1 diffractometer. SEM and EDS measurements were made on a LEO-1530 microscope. After a metal oxide deposition, round electrode of 0.5 inch in diameter were cut out from the porous films and used to construct supercapacitor coin cells. All coin cells consisted of 2 electrodes separated by a cell guard separator each connected to a current collector and enclosed in a 2016 stainless-steel coin cell. Electrochemistry measurements were carried out on these cells using a Solartron potentiostat coupled with a Solartron frequency response analyzer.
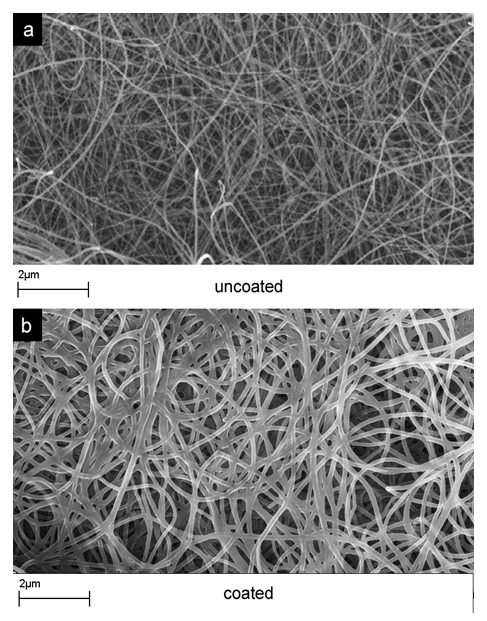
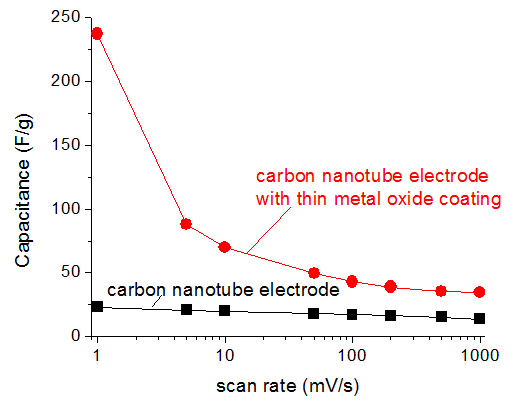
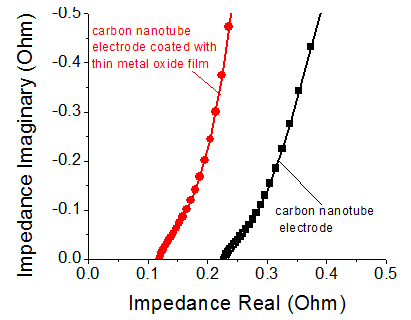
EDS and SEM measurements revealed that optimized deposition process yielded uniform metal oxide coating layer on carbon nanotube electrodes (Figure 1) and highly none-uniform metal oxide coating on the surface of the acetylene black electrodes (not shown). Electrochemical tests reveal significant enhancement in both the specific capacitance of the electrodes (Figure 2) and resistance (Figure 3) of the cells after metal oxide depositions. Even for coatings thicker than 50 nm (Figure 1 b) specific capacitance of the coated electrode exceeded that of the initial carbon, particularly at low scan rates (Figure 2) when more time is available for the ion diffusion within the metal oxide films. Improvement in the resistance (Figure 3) was revealed to be due to the improved wetting properties of the coated electrode in the cell electrolyte.
The conducted research was critical for the PI career as it revealed the importance of the fine control over the structural properties of the nanocomposites in order to achieve the breakthrough performance in energy storage devices.
The results of this work was covered in several international meetings: (1) A. Magasinski, B. Hertzberg, Y. Korenblit, I. Kovalenko, A. Kajdos, G. Yushin, Novel Materials for Advanced Supercapacitors and Li-ion Batteries, The 76th Annual Meeting of the Southeastern Section of the American Physical Society, Atlanta, GA (2009); (2) B. Hertzberg, S. Boukhalfa, A. Magasinski, I. Kovalenko, P. Dixon, Y. Korenblit, G. Yushin, Carbon-Containing Nanocomposite Materials for Energy Storage, The Annual Meeting of the American Chemical Society, Boston, MA (2009).
One of the graduate students involved in this project (Yair Korenblit) has received several awards for his work, such as: (1) 1st Place Oral Presentation Award at the Annual Georgia Tech Graduate Research Symposium, 2009; (2) 1st Place Oral Presentation Award at the Annual Georgia Tech Graduate Technical Symposium, 2009; (3) 1st Place Poster Presentation Award at the Annual Georgia Tech Graduate Technical Symposium, 2009.
Inspired by the results of this project, the PI has explored a somewhat similar approach for the formation of Si anodes for high-capacity Li-ion batteries. The Li-ion work has been selected to receive funding support from NASA and was featured in Nature Materials journal (Magasinski, A. et al. High-performance lithium-ion anodes using hierarchical bottom-up approach. Nature Materials 9, 353-358 (2010).
Figure 1. SEM micrographs of carbon nanotube electrodes before (a) and after (b) transition metal oxide deposition. A uniform coating is clearly visible.
Figure 2. Electrochemical performance of carbon electrodes before (black) and after (red) metal oxide deposition. Symmetric supercapacitor cells have been used in this study. Figure 3. Electrochemical impedance spectroscopy of supercapacitors made of carbon nanotube electrodes before (black) and after (red) metal oxide deposition. Symmetric supercapacitor cells have been used in this study.
Copyright © American Chemical Society

