AmericanChemicalSociety.com
Reports: G3 48170-G3: Synthesis of Polymers for Photovoltaic Cells by Cross-Coupling Reactions Mediated by NHC-Palladium Complexes (NHC = N-Heterocyclic Carbene)
Oscar Navarro, University of Hawaii (Manoa)
The use of
polythiophenes as conducting polymers has attracted a great deal of interest
for applications in many organic electronic devices, such as field-effect
transistors (FETs), thin-film transistor (
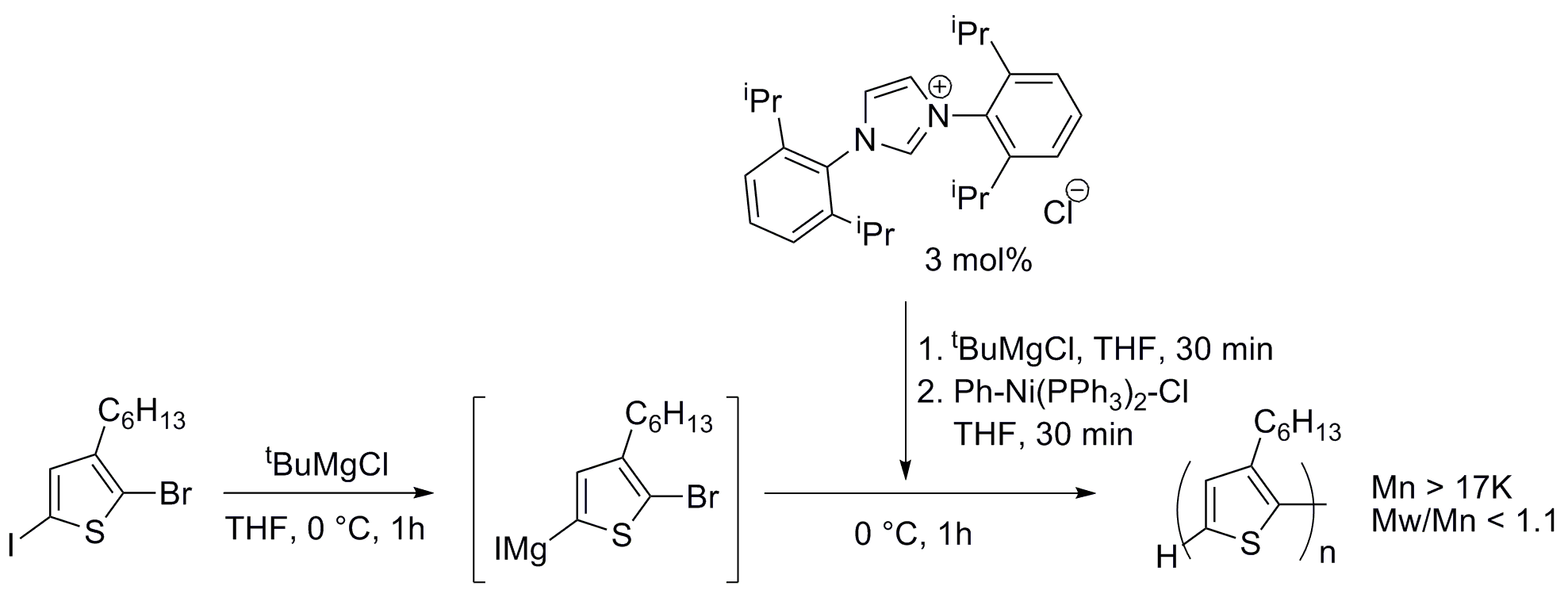
The initial goal of this project was the development of (NHC)-Pd systems for the polymerization of thiophenes using Suzuki-Miyaura cross-coupling reactions.[8] We wanted to address two main limitations of the GRIM process: low functional group tolerance and the need of preparing the Grignard reagent in situ, which requires the stoichiometry of the reaction to be precisely controlled in order to achieve high molar mass material. To obtain a preliminary confirmation of the positive effect of the use of NHC as ligands for the synthesis of polythiophenes, we decided to test first our premise in a GRIM (Ni-catalyzed) polymerization. In our previous report, we described how by modifying Kiriy's conditions6 using N-heterocyclic carbene ligands (NHC)[9] instead of the classical phosphines, we had been able to prepare high molecular weight P3HT with one of the lowest values of polydispersity reported to date for this molecular weight range (1.09). Our optimized system makes use of Ph‑Ni(PPh3)2‑Br as the pre-catalyst (2.5 mol%) in the presence of a small excess of the imidazolium salt 1,3‑bis(2,6‑diisopropylphenyl)imidazol-2‑ylidene chloride (IPr·HCl) (precursor of the NHC) at 0 °C for 3 hours (Scheme 1). Our following step was to carry out experiments to determine the catalytic active species, with the goal of designing a well-defined NHC-bearing complex to act as a pre-catalyst for these polymerizations. We were also interested in synthesizing chlorothiophene monomers to attempt the first polymerization of that type of substrates.
Scheme SEQ Scheme \* ARABIC 1. Thiophene polymerization using an
(NHC)-Ni catalyst Technical
problems with our gel permeation system (GPC-120, Agilent –former Polymer
Labs), currently out of service, have prevented us from a more exhaustive look
into our polymerization systems, and we have focused our efforts on
corroborating the results of our optimized system. In collaboration with Prof.
Michael Turner from The University of Manchester, UK, whose group performed GPC
characterization of new samples, we have been able to at least make sure that
our initial results are reproducible and not false positives due to our
malfunctioning GPC. This collaboration with Prof. Turner has also derived in a
review recently accepted for publication in Current
Organic Chemistry, and in which support from the ACS-PRF is acknowledged.
These preliminary
results have also provided us the basis for a full proposal that will be
submitted this fall to the National Science Foundation in order to address all
the goals that we wanted to achieve with this ACS-PRF grant and to expand these
couplings to a variety of substrates.
[1]. Jeffries-El, M.; McCullough, R. D. in Handbook of Conducting Polymers, 3rd ed.; Skotheim, T. A.; Reynolds, J. R.; Eds.; CRC Press Inc Taylor & Francis Group: Boca Raton, 2007, Chapter 9.
[2]. Günes, S.; Neugebauer, H.; Sariciftci, N. S. Chem. Rev. 2007, 107, 1324‑1338.
[3]. For the McCullough Method, see: (a) McCullough, R. D.; Lowe, R. D. J. Chem. Soc., Chem. Commun. 1992, 70-72. (b) McCullough, R. D.; Lowe, R. D.; Jayaraman, M.; Anderson, D. L. J. Org. Chem. 1993, 58, 904-912.
[4] For the Rieke Method, see: (a) Chen, T.-A.; Rieke R. D. J. Am. Chem. Soc. 1992, 114, 10087‑10088. (b) Chen, T.-A.; O'Brien, R. A.; Rieke, R. D. Macromolecules 1993, 26, 3462‑3463. (c) Chen, T.-A.; Wu, X.; Rieke R. D. J. Am. Chem. Soc. 1995, 117, 233‑244.
[5]. For the GRIM method, see: (a) Loewe, R. S.; Khersonsky, S. M.; McCullough R. D. Adv. Mater. 1999, 11, 250‑253. (b) Loewe, R. S.; Ewbank, P. C.; Liu, J.; Zhai, L.; McCullough, R. D. Macromolecules 2001, 34, 4324‑4333.
[6]. (a) Beryozkina, T.; Senkovskyy, V.; Kaul, E.; Kiriy, A. Macromolecules 2008, 41, 7817‑7823.
[7]. (a) Doubina, N.; Ho, A.; Jen, A. K.-Y.; Luscombe, C. K. Macromolecules 2009, 42, 7670-7677. (b) Bronstein, H. A.; Luscombe, C. K. J. Am. Chem. Soc. 2009, 131, 12894-12895.
[8]. Miyaura, N. Organoboron Compounds. Top. Curr. Chem. 2002, 219, 11-59.
[9]. (a) Hermann, W. A. Angew. Chem., Int. Ed. 2002, 41, 1290‑1309. (b) Nolan, S. P., Ed. N‑Heterocyclic Carbenes in Synthesis; Wiley‑VCH: Weinheim, Germany, 2006. (c) Glorius, F., Ed. N‑Heterocyclic Carbenes in Transition Metal Catalysis; Topics in Organometallic Chemistry, Vol 21; Springer‑Verlag, Berlin/Heidelberg, Germany 2007. (d) Díez-González, S.; Nolan, S. P. Coord. Chem. Rev. 2007, 251, 874‑883.
Copyright © American Chemical Society

