AmericanChemicalSociety.com
Reports: UNI1 49499-UNI1: Metathesis Reactions of Acyloxysulfones for Polyene Synthesis
Gregory W. O'Neil, PhD, Western Washington University
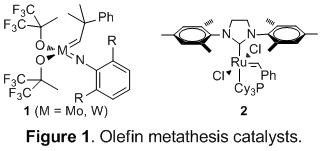
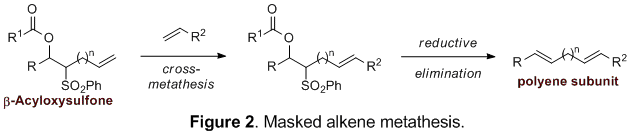
We set out to investigate the use of β-acyloxysulfones as masked alkenes, allowing for a metathesis approach to polyene subunits difficult to obtain by standard metathesis technology (Figure 2).
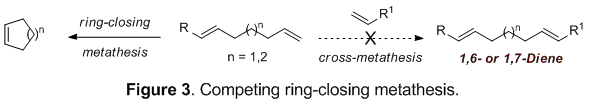
1,6- And 1,7-dienes, important substrates for a variety of cycloisomerization reactions, were chosen as initial targets for this reaction sequence. These compounds are particularly challenging to prepare by cross-metathesis due to a competing rapid ring-closure (Figure 3).
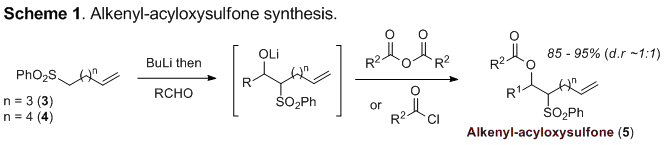
The requisite alkenyl-acyloxysulfones were conveniently prepared by the addition of lithiated sulfone 3 or 4 to an aldehyde followed by in situ acylation of the intermediate alkoxide, affording substrates of type 5 as approximately 1:1 mixtures of diastereomers (Scheme 1).
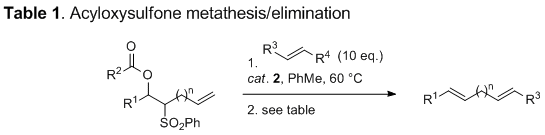
Grubbs' second-generation catalyst 2 proved capable of effecting the desired cross-metathesis, affording exclusively (E)-alkenyl-acyloxysulfone adducts as detectable by NMR. Reductive elimination then completed our metathesis approach to 1,6- (n = 3) and 1,7-dienes (n = 4) as summarized in Table 1.
This general strategy has been
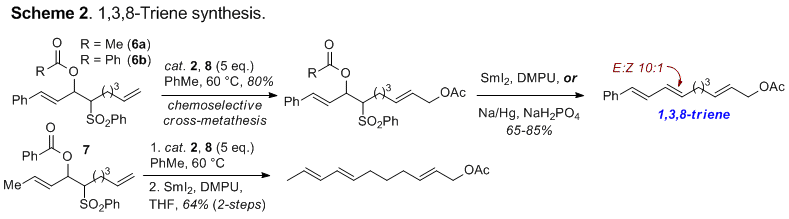
extended to include the synthesis of trienyl subunits. Based on reported
reactivity trends, it was anticipated that compounds of type 6 and 7 would undergo selective metathesis at the monosubstituted alkene
(Scheme 2). Metathesis of both the acetyl (6a)-
and benzoyloxysulfones (6b) derived
from cinnamaldehyde and crotonaldehyde (7)
with cis-1,4-bisacetylated butenediol
(8) proceeded with high
chemoselectivity. Elimination with Na/Hg or SmI2 then completed our
metathesis approach to 1,3,8-trienes. Fully
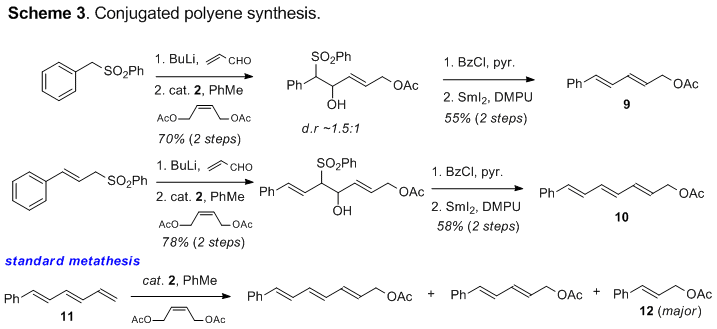
conjugated systems can also be prepared using this protocol. It was found that
metathesis was best carried out prior to acylation as depicted in Scheme 3.
Post-metathesis acylation/ elimination gave conjugated diene 9 and triene 10 in 55% and 58% yield respectively for the two steps. As a
comparison, attempted cross-metathesis with terminal triene 11 under standard conditions gave a
mixture of alkene, diene, and triene products in favor of compound 12. The
preliminary results provide proof-of-concept that a β-acyloxysulfone
metathesis/elimination strategy can provide convenient access to a number of
polyene systems. Efforts are ongoing to apply this reaction sequence to the
preparation of increasingly complex frameworks with applications in natural
product and polymer synthesis.
Copyright © American Chemical Society