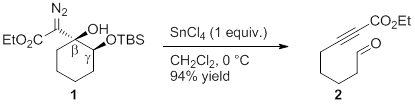AmericanChemicalSociety.com
Reports: G1 47627-G1: Fundamental Studies and Synthetic Applications of the Reaction of Hydrazones with 'Activated' Dimethyl Sulfoxide
Matthias Brewer, University of Vermont
The overarching objective of our research is to establish new synthetic organic methodology to prepare functional group-rich compounds. Recently we have been focusing our efforts on developing efficient methods to prepare polycyclic nitrogen-containing heterocycles, as well as medium sized rings and macrocycles. To accomplish this goal we have been further investigating our discovery that cyclic γ-silyloxy-β-hydroxy-α-diazo carbonyls (e.g. 1, Scheme 1) undergo Lewis acid-promoted fragmentation of the Cβ-Cγ bond to give tethered aldehyde ynoate (or ynone) products. Our discovery of this fragmentation is important because there are few synthetically useful fragmentation reactions known, and none that provide the aldehyde ynoate functional group combination. The fragmentation reactions that are known hold a special position in organic synthesis because they can unmask latent functional groups under chemoselective reaction conditions and they can provide functionalized synthetic intermediates that are otherwise difficult to prepare.
Scheme 1
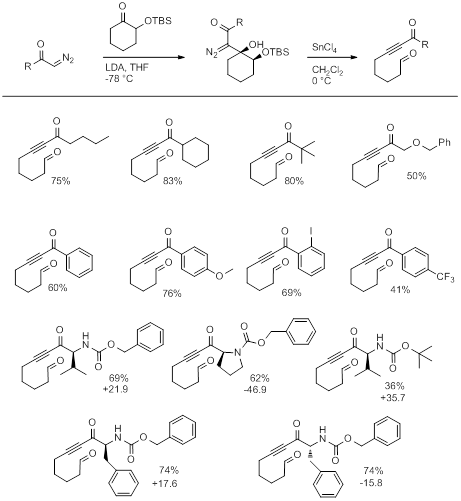
With the support of PRF funding, we have been able to further explore the scope of this fragmentation reaction and apply it toward the synthesis of tethered aldehyde ynones (Table 1). The utility of this method stems from the fact that the fragmentation precursors are easy to prepare by the aldol type addition of a lithiated α-diazo ketone and an α-silyloxy ketone. Importantly, the requisite α-diazo ketones are easy to make from carboxylic acids and thus a wide range of fragmentation precursors can be prepared.
Table 1: Preparation of tethered aldehyde ynones
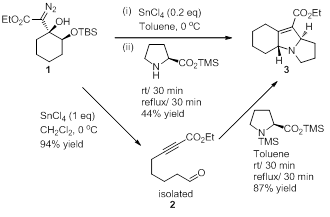
With the scope of the fragmentation established, we have turned our attention to utilizing the fragmentation products in subsequent reactions. Recognizing that intramolecular reactions are an efficient and effective way to quickly build structural complexity, we have begun exploiting the unique functional group combination present in the above fragmentation products in intramolecular reactions. Because of the predominance of polycyclic nitrogen containing heterocycles in biologically active compounds we have focused our studies on methods to efficiently prepare these scaffolds. Specifically, we have identified that polycyclic 2,5-dihydropyrroles, a heterocycle framework found in many biologically active compounds, can be efficiently prepared from the above tethered aldehyde ynoate fragmentation products by an intramolecular 1,3-dipolar cycloaddition. For example, diazo 1 was converted to tricyclic 2,5-dihydropyrole 3 (Scheme 2) in 44% yield by a one-pot fragmentation / azomethine ylide 1,3-dipolar cycloaddition sequence, and the yield of 2,5-dihydropyrrole 3 improves to 83% over two steps when the fragmentation product is isolated prior to dipolar cycloaddition. This sequence of reactions is illustrative of our goal, which is to develop efficient routes to structurally complex heterocycles from simple precursors. In this case a commercially available α-hydroxy ketone was converted into a much more structurally complex tricyclic nitrogen-containing heterocycle by a short and high yielding sequence of steps.
Scheme 2
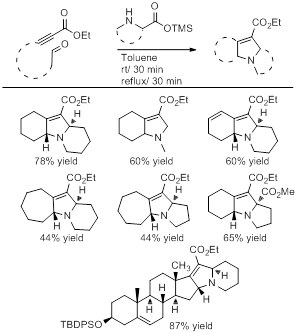
This reaction sequence appears to be general and by varying the amino acid and the tethered aldehyde ynoate we successfully prepared a variety of 2,5-dihydropyrroles, some of which are shown in Table 2. With these preliminary results in hand, we are now focusing our efforts on applying the ring fragmentation / 1,3-dipolar cycloaddition sequence to prepare structurally complex natural products.
Table 2: Polycyclic 2,5-dihydropyrrole products
Finally, PRF's support of this work has also allowed us to explore the possibility of applying this fragmentation methodology to the preparation of medium and large ring systems. Compounds that contain medium-sized-rings and macrocycles are highly effective antibiotics, anti-cancer agents, antifungals, immunosuppression agents, antiparasitics, insecticides, and immunomodulators. The high biological activity exhibited by large ring compounds is thought to derive from the fact that these structures have a desirable balance between structural pre-organization and flexibility. That is, there is sufficient flexibility to allow the compound to adopt a requisite binding geometry, but sufficient rigidity such that adopting this geometry does not have a large entropic cost. Unfortunately, current limitations of the synthesis of medium and large rings has hindered a thorough study of these systems. There is growing interest in exploring macrocycles and new methods are needed to efficiently prepare these challenging targets.
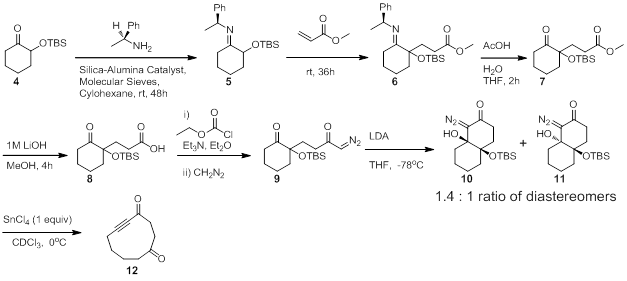
Our hypothesis was that bicyclic γ-silyloxy-β-hydroxy-α-diazo carbonyl compounds in which the β-γ bond is the ring fusion bond of the bicyclic system would fragment to provide medium and large cyclo-2-ynone products. In order to test our hypothesis we prepared bicyclic diazos 10 and 11 by the route shown in Scheme 3. We were interested to observe that the LDA-mediated intramolecular cyclization of diazo 9 provided the desired bicyclic fragmentation precursors as a 1.4 to 1 mixture of separable diastereomers. With the requisite fragmentation precursors in hand, we were able to subject both diastereomers separately to the fragmentation conditions. In the event, treating diastereomers 10 and 11 separately with SnCl4 resulted in very clean reactions that provided the same desired ynone product 12. Proton NMR of the crude reaction mixtures appeared very clean with little to no side products and GC/MS analysis showed essentially pure material with the correct mass. We currently in the process of evaluating the scope and generality of this large-ring forming reaction.
Scheme 3
To date, this work has resulted in the publication of a JACS communication, a JOC Note, and a JOC full paper that was chosen by the editor to be a Feature Article. Each of these papers acknowledges PRF's support of this work, which was integral to the success of this project. This award has had a large impact on my career in that it provided me with the requisite seed money that was necessary to generate the preliminary results required to successfully compete for funding in the form of a NIH-R01 grant. Support of this work has also had a large impact on the careers of the graduate and undergraduate students who have worked on these projects. This research has been an excellent mechanism to train these developing scientists to become independent researchers; the students who worked on these projects have received training in the general areas of reaction design and development, organic synthesis and complex structure elucidation and characterization.
Copyright © American Chemical Society