AmericanChemicalSociety.com
Reports: B3 48504-B3: Synthesis, Characterization, and Reactivity of Platinum/Ruthenium Heterometallic Complexes
Nancy Carter Dopke, Alma College
Progress has been achieved in three distinct arms of the overall project studying the influence of structural changes in platinum-ruthenium complexes on the reactivity with alcohols in atmospheric oxygen. Three undergraduate students did research during academic terms and two worked during the summer on the synthesis of heterometallic complexes. The majority of the progress was accomplished during the summer.
Previous work on the project established the synthetic procedure and full characterization of [(dppePt)2(m3-S)2Ru(PPh3)2Cl]Cl (dppe = 1,2-bis(diphenylphosphino)ethane). One student's current work is focused on synthesizing the analogous compound where the three metal atoms are held by only one bridge sulfur atom. The phosphine ligand has been changed to dppp (1,2-bis(diphenylphosphino)propane) for easier comparison to NMR literature values. The synthesis of the starting monomer dpppPtCl2 is achieved in yields up to 66% using a microwave reactor. After struggling with side reactions due to the solvent used in the monomer reaction, the complex (dppp)2Pt2(m-S)2 has been cleanly synthesized and then subjected to reductive desulfurization. The resulting reactive complex (dppp)2Pt2S was immediately reacted with Ru(PPh3)3Cl2. The resulting solid is being characterized.
This student has also been finalizing the investigation into the use of a microwave reactor in the synthesis of monomer platinum complexes. The monodentate ligand PPh3 presented the challenge of the possibility of cis and trans isomers for (Ph3P)2PtCl2. Reaction time and solvent choice can lead to a pure cis product. A manuscript describing this work is currently in preparation for submission to Inorganica Chimica Acta.
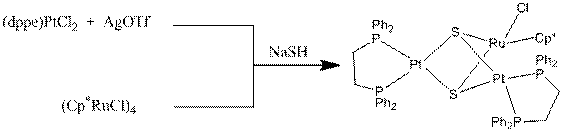
Another student has been exploring the synthesis of
heterometallic complexes using platinum monomers as the starting material. The reaction between dppePtCl2 and
the ruthenium cube species (Cp*RuCl)4
with NaSH yields a product that has been
characterized by NMR and MALDI MS.
(Thank you to Dr. Doug Benson at
Reactions using (PPh3)2PtCl2
as the platinum starting material yield an analogous complex. Further characterization and studies of the
complexes' reactivity are underway.
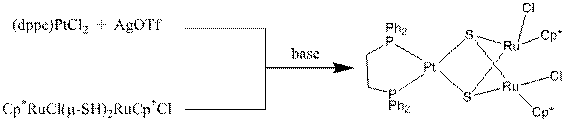
The ruthenium rich complex (dppe)Pt(m3-S)2(RuCp*Cl)2, synthesis
below, has been further characterized and the synthesis is reproducible.
With weather changes outside and the necessity to move our
set up, we found that we were increasingly having difficulties with solvent
loss in our catalytic runs. We designed
a single piece glass apparatus for the runs that decreases solvent loss and is
easier to use with our oil bath. In
addition, we changed from neopentyl alcohol to the
more reactive 1-phenylethanol so that our NMR integration analysis would be
less prone to noise in our experiment.
The grant allowed the purchase of the apparatus.
Funds from the grant made it possible for two undergraduate
students to contribute to the project over the summer. An undergraduate researcher presented a poster
titled “Microwave-assisted syntheses of
bis(phosphine)platinum(II) complexes” at the March 2010
National American Chemical Society meeting in
Copyright © American Chemical Society