AmericanChemicalSociety.com
Reports: AC8 47713-AC8: Effect of Pore-size Controlled Solubility on Mineralization, Porosity, and Permeability in Porous Media: a Combined Experimental and Theoretical Study
Jay J. Ague, Yale University
Introduction
The pore-space in rocks, soils, and other porous media typically comprises pores which range in size from millimeters to nanometers. Although macro-porosity is usually considered to dominate transport and flow in porous media, nano-scale pores often make up a significant proportion of the total porosity, playing an important role during diagenesis and mineralization. In this project, we examined the impact of pore-size controlled solubility (PCS) on mineral precipitation in geological media; the process enables fluids in micron to submicron-scale pores to reach high levels of supersaturation relative to fluids in bulk systems and may therefore be a central factor in determining patterns of mineral precipitation and dissolution in geological formations. Crucially, while PCS has implications for a wide range of geological and industrial systems, few studies have attempted to assess its importance in natural settings. The main objective was therefore to quantitatively evaluate the impact of small-scale pores on mineral solubility and crystal growth in natural geological systems by comparing field data with numerical models of mineralization. Experimental techniques to quantify the mechanism in the laboratory were also developed.
Modeling
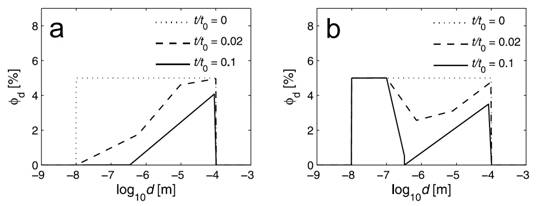
We developed a new one-dimensional numerical model, using a moving boundary condition, to describe the effect of mineralization in the rock matrix adjacent to a pressure solution (stylolite) interface. In the model, the initial polymodal pore size distribution is allowed to change in response to mineral precipitation. Porosity and pore size evolution was simulated for systems with constant mineral solubility and for systems in which solubility was pore size controlled. Total porosity decreases near the stylolite interface in all the simulations. However, in systems with constant solubility, nanometer-scale pores close rapidly because of their high specific surface area (Figure 1a); by contrast, when the PCS mechanism is included in the model, transient bimodal pore size distributions develop (Figure 1b), with submicron pores remaining open throughout the simulation. Our simulations suggest that the combination of PCS with kinetic models for mineral precipitation can account for the bimodal pore size distributions observed in some fine-grained sedimentary carbonate rocks. Different behavior is predicted for sandstones (see below).
Figure 1: Partial porosity as a function of pore size at the stylolite interface at different times for (a) the constant solubility model and (b) the PCS model. Note the development of a bimodal distribution in the PCS model.
Field measurements and model verification
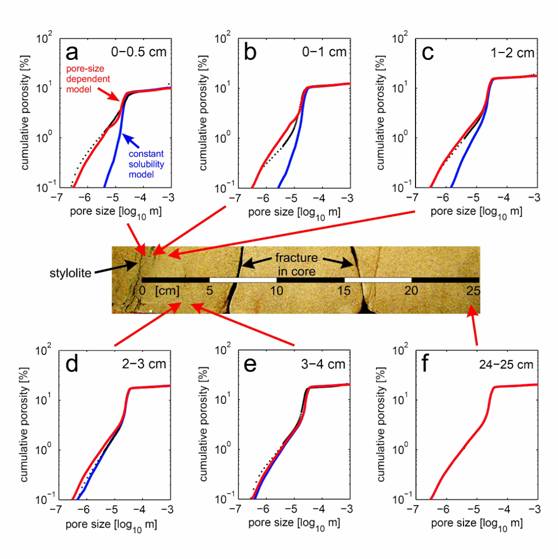
We extended and tested the model by measuring pore size distributions in a naturally-cemented sandstone adjacent to a stylolite. We found that quartz precipitation was inhibited in pores smaller than ~10 microns in diameter. In addition, we demonstrated that standard kinetic formulations cannot reproduce the observed pore-size patterns in mineralized samples, while excellent fits with the data are obtained when PCS is accounted for (Figure 2). Furthermore, we propose that reduced reaction rates in porous media with micron- to submicron-scale porosity may help explain the anomalously slow reaction rates often observed in geological systems.
Figure 2: Measured and simulated cumulative porosity curves at 6 distances from the stylolite. Measured porosity (black circles) and model curves (blue - constant solubility model, red - pore-size dependent solubility model) are given as a function of pore diameter. In (a)-(c), the pore-size dependent solubility model is in excellent agreement with the measured data, while the constant solubility model fails. The cutoff at 10-3 m reflects the upper limit of the pore-size measurements.
Relation to weathering rates
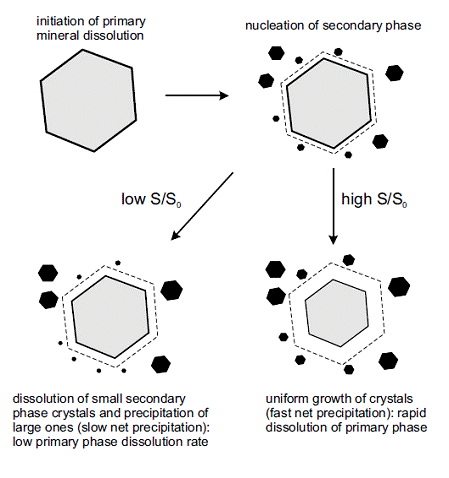
By extending some of the concepts of pore-size controlled solubility to weathering systems, we have developed a new approach to quantify the role played by interfacial energy, crystal size, and degree of supersaturation on precipitation kinetics. We demonstrate that net mineral precipitation rates in systems that are close to equilibrium, and which possess a large number of micron and nanometer scale crystals, can be much lower than rates predicted by standard kinetic equations. As primary dissolution rates are determined by the rate of secondary mineral precipitation, we propose that interfacial energy effects may account for natural weathering rates of primary minerals that are often orders of magnitude lower than the rates of mineral dissolution measured in laboratory experiments.
Figure 3: Schematic representation of primary mineral weathering and secondary phase formation. Low supersaturation (S/S0) is likely to occur during natural weathering of minerals, while high supersaturation often characterizes laboratory experiments.
Laboratory experiments
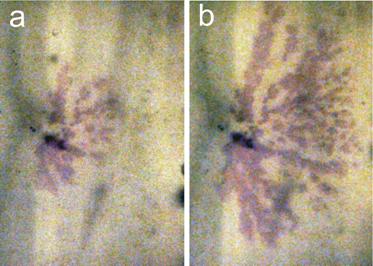
Experiments in flow through cells were conducted to test for the PCS effect. Cells were packed with natural porous material (diatomite) and mineral precipitation was induced by creating internal temperature gradients in the cell. However, for mineral precipitation to be observed on laboratory time scales, high levels of supersaturation were necessary. As a result, mineralization filled all pores regardless of size; while this meant that we were unable to observe the PCS mechanism, a dendritic crystal growth within the porous medium occurred (Figure 4). Such mineral structures were not anticipated, and experiments are being carried out to determine the factors controlling the evolution of dendrites in porous media.
Figure 4: Dendritic growth of alum in a diatomite matrix (a) after approximately 12 hours and (b) 24 hours.
Study implications and ongoing work
Our study demonstrates that PCS can be an important mechanism in geological media; by suppressing mineral precipitation in very small pores, PCS can reduce overall reaction rates by orders of magnitude. Thus, our study has wide reaching implications for a wide range of geological, environmental, and industrial processes involving the evolution of porosity and permeability, in particular hydrocarbon recovery and CO2 sequestration. Additional experiments, field work and theoretical work are being planned to further evaluate the PCS mechanism under different conditions. To date, two papers have been published, one has been submitted, and one is in preparation. In addition, an invited book chapter summarizing some of our results is near completion and four conference abstracts have also been submitted.
Copyright © American Chemical Society