AmericanChemicalSociety.com
Reports: B3 43124-B3: Magnetic Studies and Room Temperature Carbon-Carbon Bond Cleavage in Ruthenium Carboxylate Clusters
Laura E. Pence, University of Hartford
The oxo-bridged
basic triruthenium carboxylate
species,
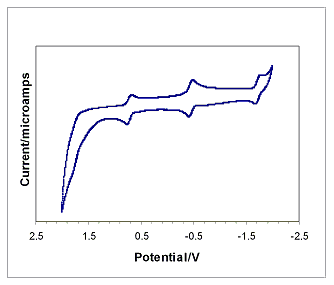
The cyclic voltammetry of the species revealed the expected reversible one electron processes. Both the isobutyrate and n-butyrate clusters display three widely spaced reversible one-electron couples and a fourth poorly resolved process as seen in Figure 1. Compared to the acetate data collected under similar conditions, the potentials of the reductions from an overall cluster charge of +1 -> 0 (-0.23 V) and 0 -> -1 (-1.50 V) are noticeably more negative, corresponding to the increased pKa of the butyric acids. In contrast, the reduction potential for the +2 -> +1 cluster charges is consistently around 0.95 V for all of the clusters, indicating that the pKa effect is negligible for electron transfer between the higher oxidation states.
Figure 1. Cyclic voltammetry
for The paramagnetism
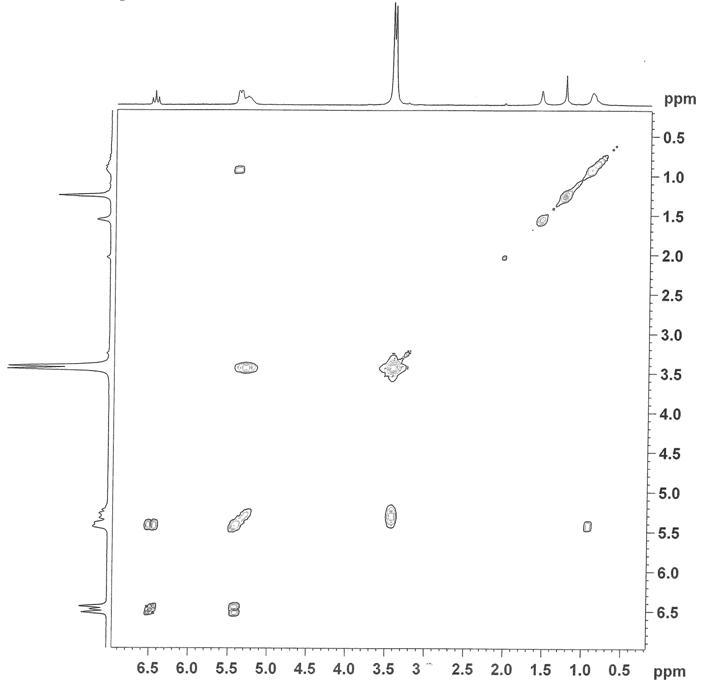
of the metal core does not preclude characterization of the complex by 1H
NMR, but it does result in several unusual features of the spectra for both the
n-butyrate and isobutyrate systems. Closer proximity of the protons to the
ruthenium atoms correlates with a relaxation of the proton signal so that the
splitting patterns become increasingly less distinct. Specifically for the ortho
proton on the axial pyridine ligands, the signal is
not only relaxed, but it is also shifted dramatically upfield
to approximately 1 ppm. The assignment of this resonance to the ortho pyridine proton was confirmed by COSY NMR, as seen in
Figure 2. Figure 2. COSY 1H
NMR Our efforts to optimize the
synthesis have correlated with the report by Toma and
coworkers,11 that this apparently simple
system is actually extremely sensitive to pH, even when carried out in organic
solvents. By using a generous amount of
acid in the first step and by minimizing the pyridine in the second step, the
desired blue complexes may be produced reliably. Our experiments have determined that in the
presence of less acid in the first step and more base in the second, that a
green product, hypothesized to be the neutral complex, Over the course of this grant award, six undergraduate
students have been supported and have greatly benefited from the experience of
doing research. Of these students, one
has gone to medical school, one earned a Masters of Public Health degree, one
earned a Masters degree in Forensic Science, and one is entering graduate
school in chemistry this fall. Although
no published manuscripts have resulted from this research, six undergraduate
research presentations have given the students a forum to hone their speaking
skills, weekly group meetings have encouraged the students to think on their
feet, and writing skills have been improved through semester end reports and
one honors thesis. As the PI of the
project, the award has had significant impact on my own career since it was an
important component of my promotion to the rank of full professor in 2009. References 1.
Baumann, J.; Salmon, D.; Wilson, S.; Meyer, T.; Hatfield, W., Inorg. Chem., 1978, 17, 3342. 2.
Abe, M.; Sasaki, Y.; Yamaguchi, T.; Ito, T., Bull. Chem. Soc. Jpn., 1992, 65, 1585. 3.
D. 4.
S. Ye, W. Zhou, M. Abe, T. Nishida, L. Cui, K. Uosaki, M. Osawa and Y.
Sasaki, J. Am. Chem. Soc., 2004, 126, 7434-7435. 5.
M. Abe, A. Sato, T. Inomata, T. Kondo, K. Uosaki and Y. Sasaki, J.C.S. Dalton Trans., 2000, 2693-2702. 6.
M. Abe, T. Kondo, K. Uosaki and Y. Sasaki, J. Electroanal. Chem., 1999, 473,
93-98. 7.
H. E. Toma, F. M. Matsumoto and C. Cipriano, J. Electroanal. Chem., 1993, 346,
261-270. 8.
S. Cosnier, A. Deronzier and A. Llobet, J. Electroanal. Chem., 1990, 280,
213-219. 9.
S. H. Toma and H. E. Toma, Electrochem.
Comm., 2006, 8, 1628-1632. 10. C. C. Pink,* N. L. Saad,*
Mugge, A. M.*; J. M. Schlough,*
Svenson, A.*; J. L. Eglin, Pence, L. E., manuscript
in preparation. 11. Nunes, G. S.; Alexiou, A. D. P.; Araki, K.; Formiga,
A. L. B.; Rocha, R. C.; Toma, H. E. Eur. J. Inorg. Chem.
2006, 1487-1495.
Copyright © American Chemical Society