AmericanChemicalSociety.com
Reports: UR1 49441-UR1: Gold(I)-Catalyzed Aromatic Claisen Rearrangements
James R. Vyvyan, PhD, Western Washington University
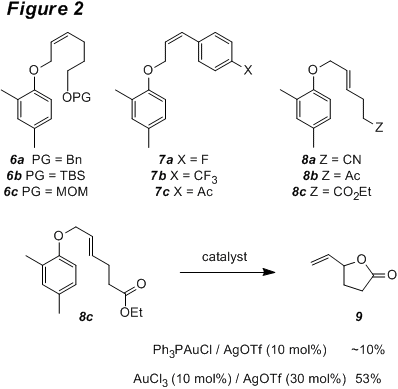
During the initial grant period, experiments were conducted to shed light on both the mechanism and scope of the Au-catalyzed Claisen-type rearrangement of allyl aryl ethers. The allyl aryl ether substrates are readily prepared by the Mitsunobu etherification of (commercially available) phenols with allylic alcohols. A significant amount of effort was expended investigating various gold catalysts, silver salts, and solvents to determine optimal reaction conditions for the rearrangement. Representative examples of the transformation using the optimized reaction conditions are shown in Figure 1. As can be seen from these simple examples, the product distribution is a function of alkene substitution and geometry. Our studies also show that the steric bulk of the allylic substituent is a key variable in determining product distribution as well. Crossover, intermolecular trapping, and stereochemical transfer experiments all support in ionic mechanism for the transformation.
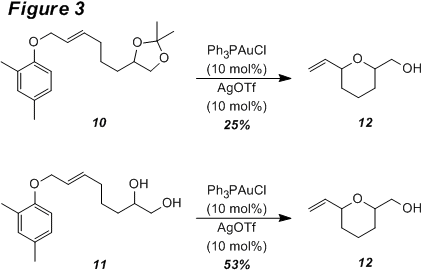
We examined substrates containing some common alcohol
protecting groups (6) in the
transformation to ascertain their compatibility with the reaction conditions
(Figure 2). Only the benzyl ether (6a) gave appreciable amounts of
rearrangement product that contained an intact protecting group. The TBS and MOM ethers (6b and 6c) were more
labile, although some rearrangement product with the free alcohol was obtained
from those substrates. Reaction of
substrates with electron poor aromatic rings conjugated to the allyl ether (7) resulted in little to no conversion
under the standard conditions. This also
supports an ionic mechanism involving an allyl cation intermediate. Substrates 8 with various functional groups remote from the allyl ether were
tested. The nitrile- and ketone-containing
substrates 8a and 8b gave low conversion, and only small
amounts of rearrangement products could be isolated. Reaction of ester 8c, however, produced lactone 9. Use of the more Lewis acidic Au(III) catalyst
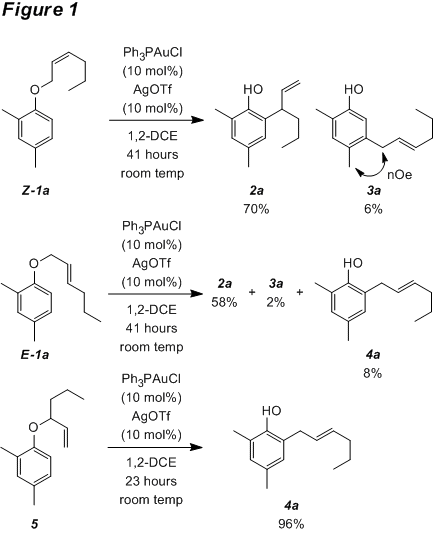
system resulted in higher yields of 9. Reaction of an allyl ether that contains a pendant acetonide group (10) under the standard conditions led to the formation of
tetrahydropyran 12 (Figure 3). Removal of the acetonide protecting group to
provide 11 prior to the
rearrangement reaction increased the yield of 12 substantially.
Functionalized tetrahydropyrans are important synthetic building blocks,
and we intend to pursue this line of investigation further in the next grant
period. Other activities in the next
grant period will involve exploring further the mechanism of the transformation
and applying the transformation to the preparation of specific synthetic
targets.
Copyright © American Chemical Society