AmericanChemicalSociety.com
Reports: AC3 47472-AC3: Heterolytic Activation of Hydrogen Promoted by Ruthenium Nanoparticles on Poly(vinylpyridine) and Hydrogenation of Aromatic Compounds
Roberto A. Sanchez-Delgado, City University of New York (Brooklyn College)
This project aims at synthesizing new nanostructured catalysts composed of metallic nanoparticles immobilized on basic supports like poly(4-vinylpyridine) or magnesium oxide. We expect the resulting nanostructures on these solids to be capable of promoting the heterolytic activation of hydrogen and the ionic hydrogenation of aromatic and heteroaromatic compounds of relevance to the manufacture of cleaner fossil fuels. Although such ionic mechanisms are well know in solution, they are extremely rare on solid surfaces.
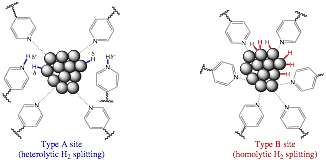
During the second year of the project a material containing 10% Ru (average size 3.1 nm) on poly(4-vinylpyridine), characterized during the first period of the grant, was optimized as a catalyst for the hydrogenation of a variety of aromatic and nitrogen-heteroaromatic molecules; it was also found that the catalyst can be re-used several times without any appreciable loss of activity. The hydrogenation rates for e.g. quinoline were strongly sensitive to solvent polarity and to the addition of external acid or base, in agreement with an ionic hydrogenation mechanism, while the rates of toluene hydrogenation were insensitive to the same factors. This suggests the existence of two different hydrogenation sites on the catalyst, one specific for polar N-heteroaromatics and capable of heterolytic hydrogen splitting (type A) and a second one specific for benzenoid aromatics, probably acting by a conventional homolytic hydrogen splitting mechanism (type B), see Fig. 1. Further evidence for the dual-site nature of this catalyst was obtained by the selective poisoning of type B sites caused by small amounts of added thiophene, which had no effect on type A sites.
Fig. 1. Model
of dual-site structure of a Ru/PVPy hydrogenation catalyst Similar
catalysts containing Ni and Pd nanoparticles immobilized on
poly(4-vinylpyridine) were synthesized and characterized by TEM and XRD, and were
also found to be efficient for the hydrogenation of N-heteroaromatic compounds like
quinoline under moderate reaction conditions, but not for reducing simple aromatic molecules like toluene. This
suggests that the Ni and Pd catalysts only display type A sites, capabe of
hydrogenating polar N-heteroaromatics. A second generation of catalysts displaying very high
hydrogenation activities is now being developed using Ru nanoparticles imbedded
in MgO as the basic support. The
best catalytic performance has been achieved with a 1% Ru/MgO material, which
hydrogenates toluene to methylcyclohexane at 120 oC and 50
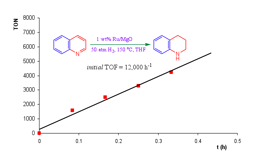
atm H2 in THF with a TOF of 1500 h-1 and quinoline to 1,2,3,4-tetrahydroquinoline
at 150 oC and 50 atm H2 with a TOF of 12,000 h-1,
probably the highest activity observed so far for the hydrogenation of
quinoline.
Fig.
2. Selective hydrogenation of quinoline by Ru/MgO An analogous
solventless reaction maintains a TOF of ca. 8500 and the catalyst shows no sign
of deactivation after reducing 50,000 mol of quinoline per mol Ru. Full characterization
of these materials, as well as optimization of
the reaction parameters and mechanistic studies is in progress.
Copyright © American Chemical Society

