AmericanChemicalSociety.com
Reports: AC9 46737-AC9: Multicomponent Droplet Growth in Supersonic Natural Gas Separators
Barbara E. Wyslouzil, Ohio State University
Natural
gas supplies ~23% of As these devices are adopted, critical
questions remain regarding droplet growth in these complex vapor mixtures. The
goal of this work is to improve our fundamental understanding of water -
hydrocarbon droplet growth at cooling rates comparable of those found in the
novel separators. In particular, hydrocarbons can inhibit water condensation
because they wet the water surface while water cannot wet the hydrocarbon
surface. Since removing water is critical to preventing clathrate
formation, understanding co-condensation in this highly non-ideal system is
vital to the success of supersonic separator technology and efficient natural
gas recovery. Our
experimental apparatus includes a series of supersonic nozzles with cooling
rates that match the supersonic separators. The characterization methods
include pressure trace measurements, Fourier transform infra-red (FTIR) spectroscopy
measurements of the gas and liquid phases, and in situ small angle x-ray scattering (SAXS) or neutron (SANS) measurements
to directly follow the growing droplets. To date we have completed an extensive
series of pressure and SAXS measurements that investigate 3 different starting
concentrations for the pure components, nonane and D2O,
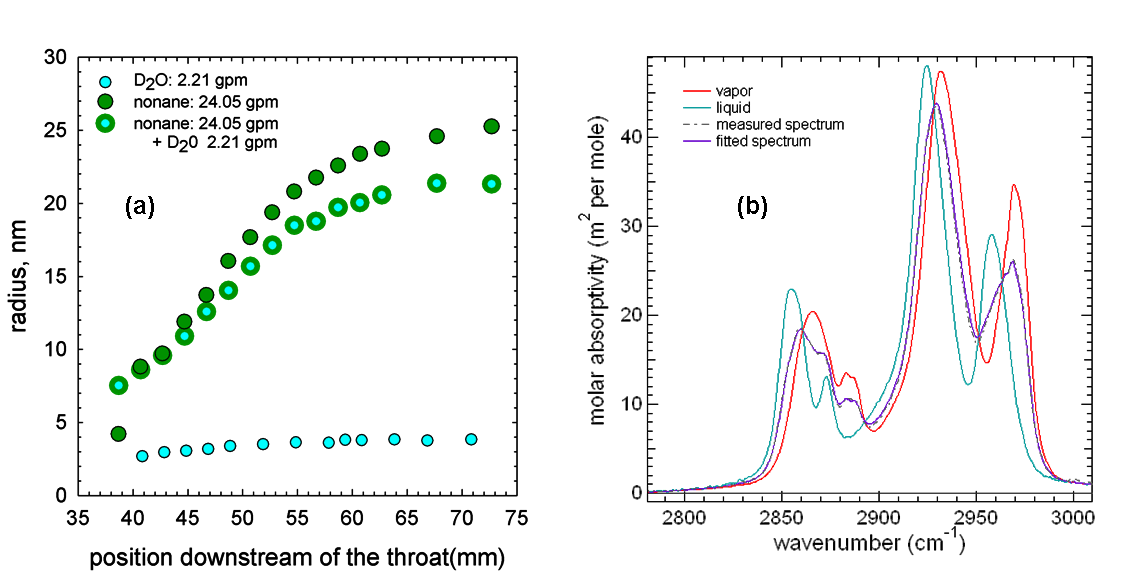
as well as the 9 corresponding binary systems. Figure 1(a) illustrates the position
resolved average droplet sizes of aerosols formed during condensation of pure D2O,
pure nonane and co-condensation of D2O nonane. Under the conditions used in these experiments, pure
nonane nucleation occurs upstream of pure D2O
nucleation, at a temperature that is about 3 K warmer. The droplets formed when
D2O is present are always smaller than the pure nonane
droplets, while the number density of the droplets formed via co-condensation
appears higher than for the pure nonane droplets (not
illustrated). This observation is surprising because under the temperatures
reached in the experiment, the formation of additional particles via D2O
nucleation is unlikely. Furthermore, the non-ideality of this system suggests
that binary nucleation should not be an efficient particle production pathway. By using D2O we can also conduct
the complementary small angle neutron scattering (SANS) measurements to better
observe the distribution of condensed water within the aerosol. Using D2O
also makes it somewhat easier to follow water condensation with FTIR spectroscopy.
We
have also completed FTIR measurements to follow condensation of the pure
components and better understand how the presence of the hydrocarbon inhibits
water removal. One challenging aspect of the work is that although the vapor
and liquid regions of the spectrum are well separated for D2O, they
are not for nonane. Nevertheless, by combining the
FTIR and SAXS data we have developed a robust method to deconvolve
the measured alkane spectra into contributions from
the vapor and the liquid. Figure 1(b) illustrates the spectra corresponding to
the vapor and the liquid, as well as the fit to a spectrum that comprises 60%
vapor and 40% liquid. The current challenge is to measure and deconvolve the spectra measured during co-condensation of
water and nonane and combine these results with the
SAXS measurements to develop a more comprehensive picture of droplet growth in
this complex system. Figure 1(a): Position resolved average droplet
sizes of aerosols formed during condensation of pure D2O, pure nonane and co-condensation of D2O nonane
under conditions where nonane condensation begins at
somewhat higher temperatures than D2O condensation. (b):
Nonane spectra corresponding to pure vapor and pure
liquid can be combined to yield a good fit to the measured nonane
spectrum.
Copyright © American Chemical Society

