AmericanChemicalSociety.com
Reports: AC3 46425-AC3: Understanding Iron Catalyzed Olefin Polymerization
Paul J. Chirik, Cornell University
Iron- and cobalt-catalyzed ethylene and a-olefin polymerization was a major breakthrough in homogeneous catalysis and has inspired numerous research groups to explore base metals as sustainable and non-toxic alternatives to precious metals. During the term of our ACS PRF-funded research program, our laboratory has been exploring the unique role of aryl-substituted bis(imino)pyridine ligands in these reactions with the goal determining the nature of the active species and ultimately the mechanism of the polymerization process. Such information is critical in the design and synthesis of new catalysts for the more efficient use of dwindling petroleum feedstocks.
We have previously reported the synthesis and full characterization of the first examples of bis(imino)pyridine iron alkyl cations, [(iPrPDI)FeCH2SiMe3][BPh4] (iPrPDI = 2,6-(2,6-iPr2-C6H3N=CMe)2C5H3N). These compounds were active pre-catalysts for the polymerization of ethylene and key in demonstrating that high spin iron compounds are competent for propagation. Monitoring the polymerization reaction with these species reveals poor initiation; only the polyethylene product and the starting iron alkyl cation are observed during turnover. During the past year, a goal in our laboratory has been to synthesize more polymerization-relevant iron alkyl cations and as well as better understand the electronic structure and ultimately the mechanism of such species.
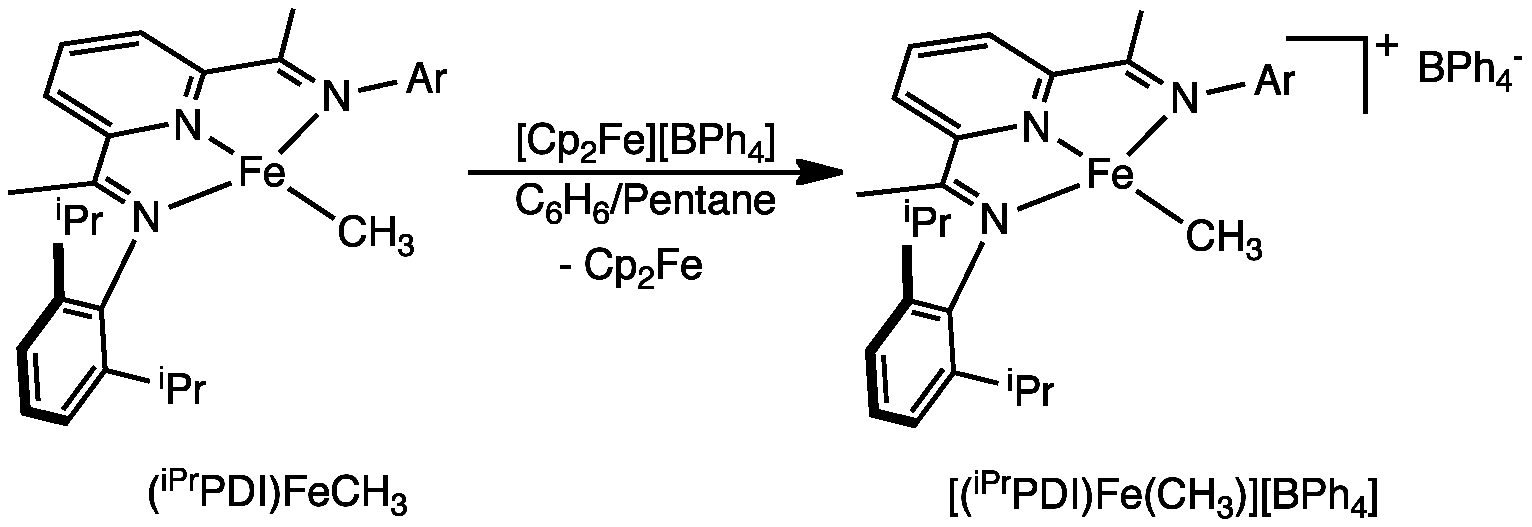
The most commonly (and unfortunately costly) used activator in homogeneous olefin polymerization catalysis is methyl aluminoxane, MAO. One function of this reaction is to convert the transition metal dihalide precursor into the active species, which is often postulated as the corresponding metal-methyl cation, e.g. [LnM-CH3]+. For this reason, bis(imino)pyridine iron methyl cations were targeted. We have discovered a new and fairly general route to these compounds. One electron oxidation of the neutral bis(imino)pyridine iron methyl complex with either [Cp2Fe][BPh4] or [Cp2Fe][BArF4] (BArF4 = B(C6H3-3,5-CF3)4) furnished the desired iron methyl cations, [(iPrPDI)FeMe]+ (Figure 1).
Figure 1. During the past year, we have also explored the electronic
structure of both the neutral and cationic iron alkyls. Previous work from our
laboratory established that the bis(imino)pyridine iron neopentyl and neosilyl
complexes, (iPrPDI)FeCH2EMe3 (E = C, Si), are S = 3/2 compounds resulting from high spin ferrous
centers (SFe = 2)
antiferromagnetically coupled to a bis(imino)pyridine radical anion (SPDI = 1/2). Upon oxidation to the corresponding alkyl
cation, the high spin ferrous oxidation state is preserved. It is important to
note that in both the neutral and cationic compounds, the alkyl ligand is
lifted substantially from the iron chelate plane. This distortion is believed
to be steric in origin and enables the high spin state at iron by reducing the s* character
of the dx2-y2
orbital.
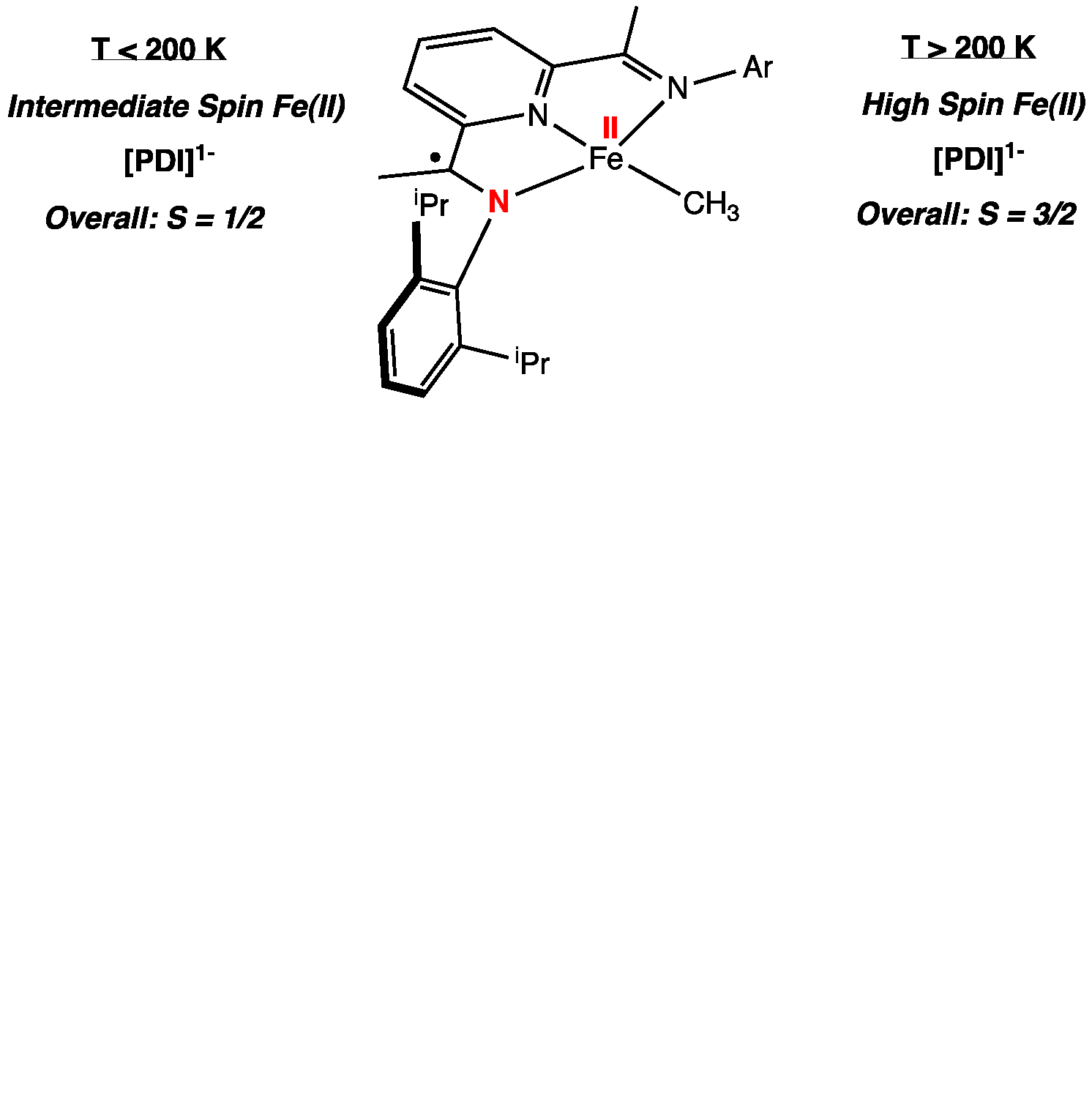
For the neutral iron methyl compound, (iPrPDI)FeMe,
more complex and interesting behavior was observed. At low temperatures, an
overall S = 1/2 compound was observed,
likely arising from an intermediate spin ferrous center (SFe = 1)
antiferromagnetically coupled to a bis(imino)pyridine
radical anion. At higher temperatures, both SQUID magnetometry and Mssbauer
spectroscopy demonstrate spin crossover to an S = 3/2 compound, analogous to the iron neopentyl and neosilyl compounds
discussed in the preceding paragraph. Notably, the solid state structures of
both the neutral and the cations establishes a planar structure, confirming the
distortions observed with the larger alkyls is steric and likely the origin of
simple paramagnetic behavior over all temperatures. Relevant to olefin
polymerization catalysis, oxidation to the iron methyl cation, [(iPrPDI)FeMe]+,
produced a high spin iron(II) with a redox innocent bis(imino)pyridine chelate.
These results demonstrate that differences in electronic structures in the
neutral iron alkyl do not translate onto the catalytic relevant cationic
species. Figure 2. The oxidative synthetic route to the iron methyl cation has
proven general among compounds with different bis(imino)pyridine ligands and
alkyl groups. We have recently assembled a library of these compounds and are
currently systematically evaluating how each of these well-defined single
component pre-catalysts functions in ethylene polymerization. Preliminary
results indicate little effect of the counter anion on the productivity of the
catalyst or in the molecular weight of the polymer produced. The new route to various bis(imino)pyridine
iron alkyl cations prompted exploration of the synthesis of related anionic
alkyl complexes to complete a series compounds that vary by three oxidation
states that will allow systematic evaluation of chelate participation. Prior to
this study, the only anionic bis(imino)pyridine iron alkyl complexes known were
[Li(THF)4][(iPrPDI)FeMe] reported by
Gambarotta and the aryl anions, [Li(Et2O)3][(iPrPDI)Fe(C6H4-4-R)N2]
(R = H, Me), synthesized by our laboratory. However, the electronic structure
of these compounds and the redox activity of the bis(imino)pyridine chelate has
not been determined. One electron reduction of the
bis(imino)pyridine neopentyl complex furnished the corresponding alkyl anion,
[Li(Et2O)3][(iPrPDI)Fe(CH2CMe3)N2],
where the reduced species also coordinated an equivalent of dinitrogen. The
electronic structure of this compound was of interest now that we have
synthesized a series of bis(imino)pyridine iron alkyl compounds that vary by
three oxidation states (Figure 3). Both magnetic and zero-field Mssbauer
spectroscopy establish a diamagnetic compound arising from a low spin ferrous
center (SFe = 0) with a
closed-shell singlet bis(imino)pyridine (SPDI = 0) dianion. X-ray diffraction revealed some of the
largest distortions to the chelate ever observed experimentally. These findings
were corroborated by DFT calculations and demonstrate that within the series of
iron alkyl complexes, the redox events are all chelate based and that the
iron(II) oxidation state is preserved. Such findings may have profound impact
on our understanding of iron catalyzed olefin polymerization.
Figure 3. One final area studied during the last funding period was
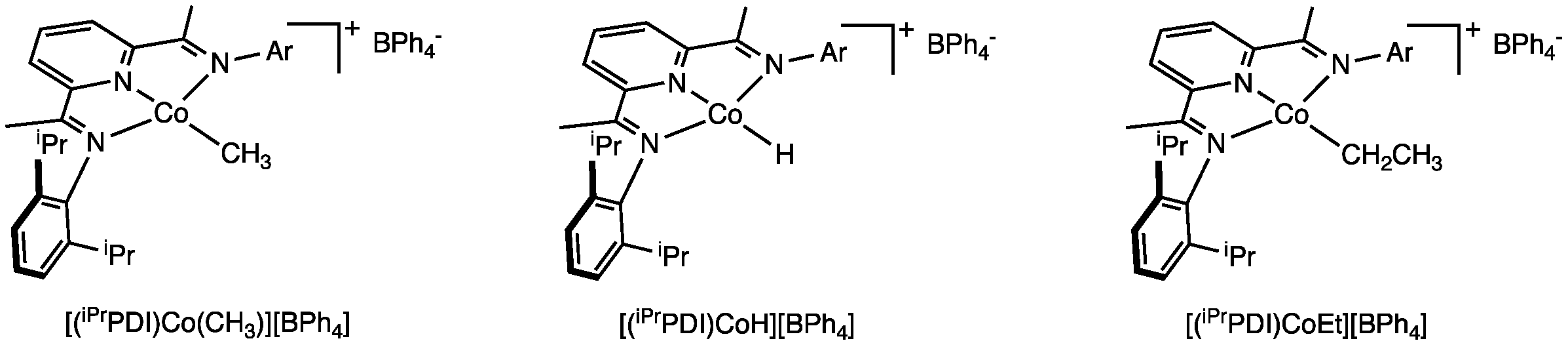
the synthesis of single component aryl-substituted bis(imino)pyridine cobalt
pre-catalysts. Using our oxidative synthetic method, several new cationic
cobalt alkyl complexes have been synthesized (Figure 4). Unlike in iron
chemistry, we have been able to access the cobalt hydride cation, [(iPrPDI)CoH]+,
as well as those containing b-hydrogens,
[(iPrPDI)CoEt]+. These species are relevant to initiating
and propagating compounds formed during turnover. Current experiments are aimed
and exploring their utility in ethylene and a-olefin
polymerization with the goal of uncovering the origin of their reduced activity
as compared to the corresponding iron compounds.
Figure 4.
Copyright © American Chemical Society