AmericanChemicalSociety.com
Reports: UNI5 49454-UNI5: Adsorption of Thiophenes at Liquid/Solid Interfaces
Katherine E. Plass, PhD, Franklin and Marshall College
The organization of molecules at solid interfaces can have a profound influence on interfacial properties and influence lubrication, catalysis, and electron transfer processes. Thiophene units are common in organic semiconductors, which are themselves incorporated into devices where performance is interface dependent. Understanding the self-assembly of these species at solid interfaces is thus of great interest. Few studies, however, have focused on uncovering the effect that the distinct molecular properties of thiophenes have on self-assembly. This grant has supported the efforts of one undergraduate student to observe the self-assembly behavior of a series simple alkyl-decorated thiophene-containing (Figure 1) using scanning tunneling microscopy (STM). Comparison of the patterns that these structurally related species form at the liquid-graphite interface is expected to provide insight into the role of various intermolecular and substrate-molecule interactions on self-assembly specific to the thiophene chemical moiety.
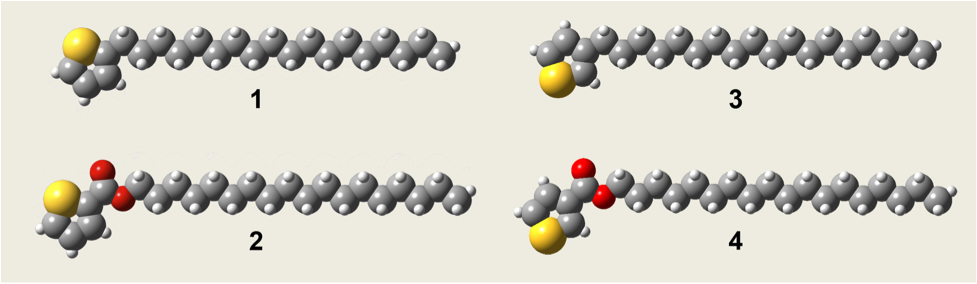
Figure 1. The
structurally related alkyl-decorated thiophene species whose self-assembly is
being investigated at the liquid-solid interface. These species differ in the
substitution position on the ring (1
versus 3 and 2 versus 4) and in the
attachment chemistry (1 versus 2 and 3 versus 4). A Franklin & Marshall College
undergraduate, Felicia Lucci, has undertaken the STM observation of the
monolayers formed by a series of thiophenes, shown in Figure 1, at the
phenyloctane-graphite interface. Each molecule contains an octadecyl chain to
slow molecular dynamics sufficiently to allow imaging on the time scale of the
STM experiment. Several modes of alkyl chain attachment chemistry were explored
to identify feasible synthetic routes that could be utilized with a variety of
commercially available precursors. In this series, the octadecyl chain is attached
to the thiophene either by a simple C-C bond (1 and 3) or via an
ester-linkage (2 and 4). The alkyl chain is bound either in
the 2 (1 and 2) or the 3 (3 and 4) position of the thiophene ring. Species
3 is commercially available. As the
synthesis and purification of species 1
and 4 has progressed, STM imaging
has been carried out on species 3
and 2 (Figure 2).
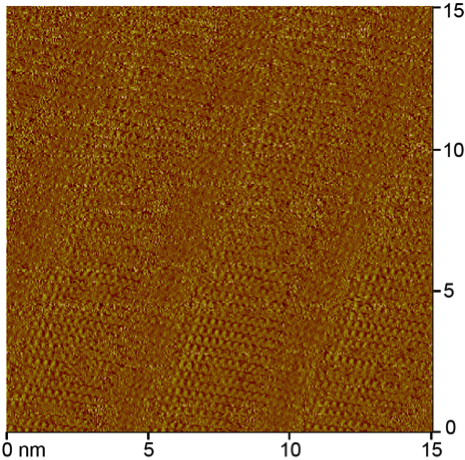
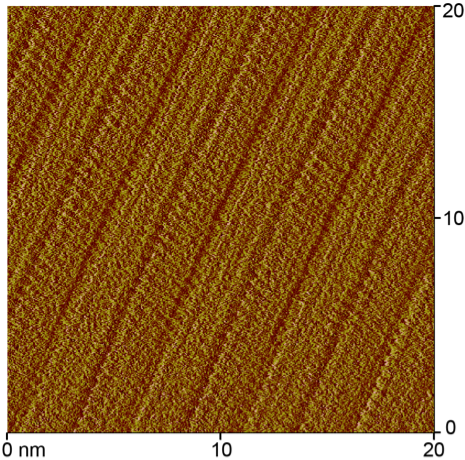
Figure 2. STM
images of the self-assembled monolayers formed at the phenyloctane-graphite
interface by species 3 (left) and 2 (right). These are current
images collected in quasi-constant height mode. The bright yellow areas denote
increased tunneling current. Comparison between the monolayers
of 2-octadecyl thiophene (3) and
2-octadecanoate thiophene (2) shows a
distinct difference in the self-assembly of these two structurally similar
species. Species 3 self-assembles
into lamellae in which the alkyl chains are at 90°
angles to the propagation direction. The alignment of the small bright spots that
represent hydrogen atoms indicates that the carbon backbone is perpendicular to
the substrate. The spacing between columns suggests that the thiophene rings of
adjacent columns face one another. This packing would optimize neither the
interactions between neighboring alkyl chains nor interactions with the
graphite substrate. As this simple 3-alkyl thiophene is most similar to the
units making up 3-hexyl polythiophene, a widely used electrically conductive
polymer, fully understanding the cause of this unexpected packing will be a
priority. The packing behavior of 2
is distinct from 3 in that there is
a greater angle between the alkyl chains and the propagation direction. A
difference in the contrast between adjacent columns may suggest an alternation
in the arrangement of the ester-containing species visible either due to the
ester functionality or the asymmetry of the ring.
These initial
results suggest that the self-assembly of alkyl-decorated thiophene-containing
species is sensitive to either the chain attachment chemistry or position, or
both. Impending imaging of species 4
will help to determine the specific impact of each factor. Detailed
computational modeling will aid in interpretation of the affect these molecular
changes have on the interactions between molecules and with the substrate. Future
work will seek to explore other modes of chain attachment to explore the effect
this has on self-assembly. The behavior
will be compared with that of structurally similar non-thiophene compounds,
furans and benzenes. This work will clarify the effects of chemical
substitution on the self-assembly of thiophenes, and provide an understanding
of the similarities and differences between thiophene self-assembly and that of
other aromatic rings.
Copyright © American Chemical Society