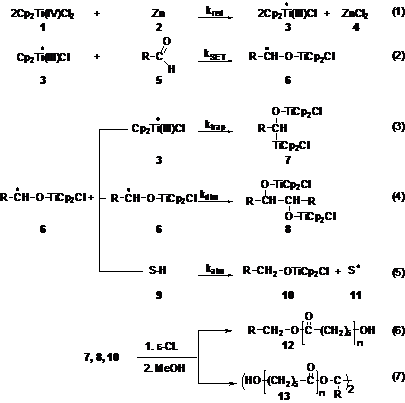47274-AC7
Early Transition Metal Catalyzed Living Radical and Ring Opening Polymerizations for Complex Copolymer Architectures
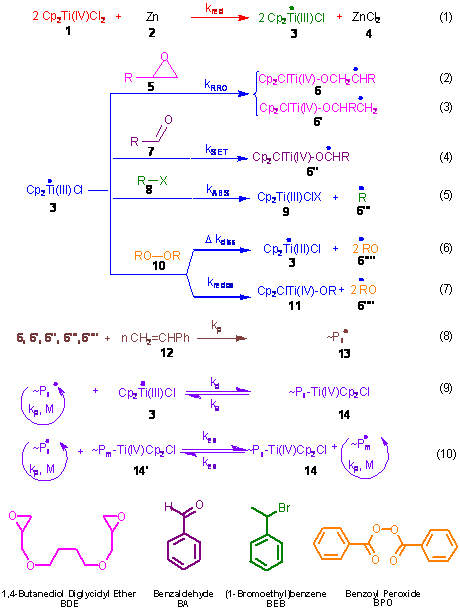
The Cp2TiCl mediated radical ring opening of epoxides, SET reduction of aldehydes, halide abstraction and redox reaction with peroxides (Scheme 1) were demonstrated as novel initiating methodologies for the living radical polymerization (LRP) of styrene. Cp2TiCl2 was shown to be the only know transition metal catalyst that can provide radical initiation from four different classes of initiators. The advantages of epoxides and aldehydes rely on their commercial availability with a wide variety of chain ends and the in situ generation of alcohol chain ends which can be further employed in the synthesis of block copolymers, whereas the halide activation provided by Ti is superior to that of Cu in ATRP since Ti can initiate even from inactivated linear alkyl halides (1-bromodecane).
Scheme 1: Cp2TiCl-catalyzed styrene LRP initiated
from epoxides, aldehydes, halides and peroxides. All
4 Cp2TiCl-activated initiator types were thoroughly comparatively
evaluated (Tetrahedron, 2008, 64, 11831) by investigating the effect of reagent stoichiometry
(monomer to initiator, Ti/initiator, Zn/Ti) and temperature on initiator
efficiency (IE) and polydispersity in the Cp2TiCl-catalyzed styrene
LRP. While living polymerization
features (linear dependence of molecular weight on conversion and low
polydispersity) were obtained in most cases over a wide range of conditions,
the comparison revealed a set of initiator specific similarities and
differences. For brevity only the
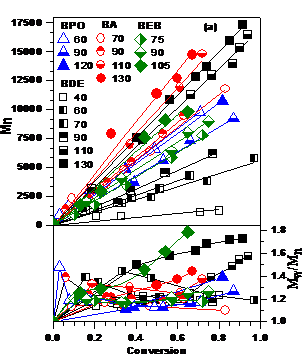
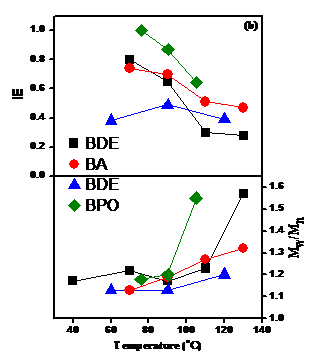
effect of temperature is presented in Figure 1. While
the polymerizations are sensitive to the initiator structure, larger initiator
efficiencies and narrower polydispersity (Mw/Mn ~ 1.2)
and are obtained when using excess Cp2TiCl over the initiator and of
Zn over Cp2TiCl2 and with decreasing temperature.
Therefore, optimum conditions which minimize PDI and maximize IE are
[St]/[I]/[Cp2TiCl2]/[Zn] = (50-200)/1/(2-3)/(4-6) at
70-90 °C. However, they are
also initiator dependent. Peroxides are good initiators, but do not provide
functional chain ends. Halides are
more sensitive to the variation in the reaction parameters and their optimum
conditions are in a narrower interval.
Finally, both epoxides and aldehydes remain synthetically the most
useful. Epoxides may provide faster
initiation and are more readily accessible on polymer backbones for the
Ti-catalyzed synthesis of block and graft copolymers. However, aldehydes not
only also allow access to the same PSt-OH functional chain ends but also seem
to be the least affected by the reaction conditions and thus the most robust
initiator in the series. Figure
1.
Effect
of temperature in the Cp2TiCl-catalyzed styrene LRPs initiated from BDE,
BA, BEB and BPO: (a) Dependence of Mn and Mw/Mn
on conversion; (b) Dependence of IE and PDI on temperature. The
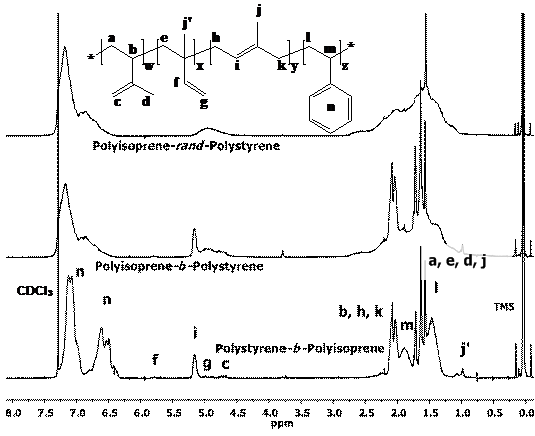
knowledge accumulated on styrene was further applied towards the isoprene LRP
since to date there is no example of a transition metal catalyzed isoprene LRP.
We have thus provided the first example of such polymerizations using again the
Cp2TiCl2/Zn system in conjunction with epoxides,
aldehydes and halides and have shown that the polymerization is living, and
that block copolymers with styrene can also be synthesized. (ACS Symposium Series, Controlled/Living
Radical Polymerization. 2009, In
Press). Figure 2. 500 MHz 1H-NMR spectra of
isoprene/styrene copolymers synthesized by Cp2TiCl-catalyzed LRP. The
first examples of the use of aldehydes in the living ring opening
polymerization of e-CL were
presented (J. Polym. Sci.: Part A: Polym. Chem. 2008, 46, 2869-2877, Scheme 2) via
the use of the SET reduction of carbonyl groups to generate in situ Ti
alkoxides. Scheme 2. Living ring
opening polymerization of
caprolactone catalyzed by titanium alkoxides derived from SET reduction
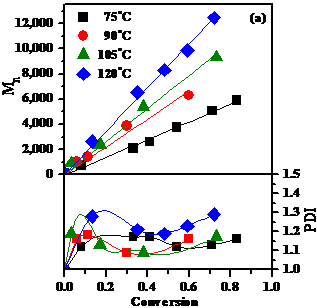
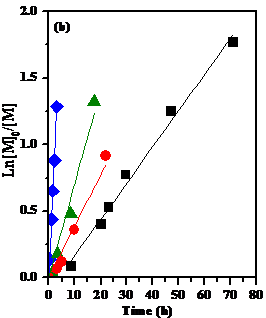
of aldehydes. Figure 2. Temperature effect in the Cp2TiCl
catalyzed caprolactone LROP (a) Dependence of Mn and PDI on
conversion; (b) First order kinetics. The
living character of the polymerization was demonstrated (Fig. 2) by the linear
dependence of Mn on conversion, low PDI values and linear kinetics,
while the aldehyde initiation was confirmed (NMR) by the presence of the
initiator fragment of the PCL chain end.
The effect of the nature of the aldehyde functionality (R-Ph-CHO, R = H,
Cl, PhCH2O, NMe2, CH3O, NO2, and
CHO), reagent ratios ([CL]/[aldehyde] = 50/1 to 400/1, [aldehyde]/[Cp2TiCl2]
= 1/1 to 1/4, [Cp2TiCl2]/[Zn] = 1/0.5 to 1/2) and
temperature (T = 75 °C to 120 °C) was
investigated over a wide range of values to reveal a living polymerization in all
cases with an optimum observed at 90 °C with
[CL]/[aldehyde]/[Cp2TiCl2]/[Zn]= 100/1/1/2. Thus,
together with epoxides, aldehydes were introduced as a new class of initiators
for the Cp2TiCl-catalyzed living ROP of cyclic esters. This convenient and inexpensive novel
methodology precludes the need for prior synthesis of air and moisture
sensitive Ti complexes and provides convenient access to PCL with variety of
chain ends derived from widely available and structurally diverse aldehyde
precursors. Current
efforts are directed towards the application of the current methodologies in
complex polymer architectures and the LRP of dienes and fluorinated monomers.