45090-GB5
Tuning Small-Molecule Permeability in Glassy Polymers with Nanoparticles
During the award period, we have made progress in both experimental work and modeling.
Experimental work:
Film preparation
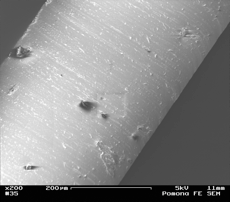
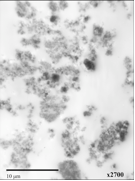
This year we focused our work on poly(dimethylsiloxane) (PDMS) membranes and PDMS-silica composite membranes, so as to examine the effects of nanoparticles in a rubbery polymer. The films were solvent-cast with long stir times, but SEM images (Figure 1) and TEM images (Figure 2) showed the presence of larger aggregates. Additionally, gas permeation experiments described below showed evidence of void formation at the PDMS-SiOx interface. Since the end of the funding term, we have obtained a sonic bath to improve dispersion. We also have begun synthesis of poly(4-methyl-2-pentyne) (PMP), an ultra-high free volume polymer, which we plan to make films from.
Figure 1. SEM image of PDMS with 10 wt% amorphous
silica nanoparticles Figure 2: TEM image of with
10 wt% SiOx nanoparticles (10 nm diameter) showing
clear agglomeration. Stšber process particles To examine effects of a wide range of impermeable particle
sizes, we have continued our work synthesizing silica particles using the
Stšber process. During our preliminary attempts last year, the particles
obtained were not spherical and were highly agglomerated. However, this year we
were able to prepare monodisperse SiOx particles with
diameters ranging from 150 to 200 nm, as shown in Figure 3 below (samples were
also analyzed via Particle Size Analysis to determine the polydispersity index,
which was close to 1 in all cases). We are currently working to broaden the
range of particle sizes using a "1-step" approach characterized in the
literature.
Figure 3. SEM image of SiOx
particles formed via the Stšber process Permeation experiments Over the 2007-2008 year, we established the reproducibility
of gas permeation experiments in our apparatus, and also ran comparative
permeation tests on the pure PDMS and PDMS/10 wt% SiOx
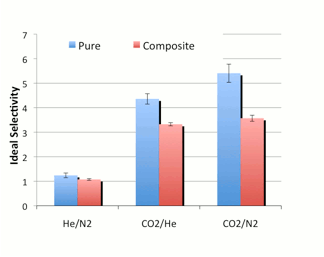
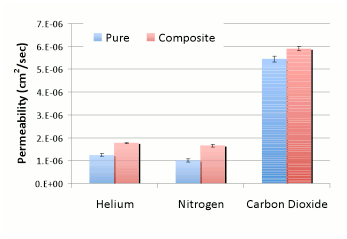
composite membranes. As shown in Figure 4a below, the permeability of the three
test gases, helium, nitrogen, and carbon dioxide, increased upon addition of
the SiOx to the PDMS films. However, Figure 4b shows
a decrease in ideal selectivity (the ratio of pure gas permeabilities),
most likely due to the formation of non-selective voids. As mentioned above,
the particles had also formed large aggregates; breaking up these aggregates
may also improve the selectivity of these films.
a) b) Figure 4:
Permeability (a) and ideal selectivity (b) of helium, nitrogen, and carbon
dioxide in pure and 10 wt% SiOx composite PDMS
membranes. Positron-Annihilation Lifetime Spectroscopy (PALS) To determine the free volume in polymers and polymer
composites (an important indicator of transport properties), we will run PALS
on the films. We previously worked with Drs. Allen Mills and Maurizio Biasini at the University of California, Riverside to
design a PALS apparatus for our work.
Although we did set up a preliminary apparatus at UCR, the Mills lab
moved locations and we were unable to complete further studies. We plan to set
up the apparatus again, and Dr. Mills has generously agreed for use to move the
apparatus to HMC. Additionally, Dr. Sergei Nazarenko
at the University of Southern Mississippi has agreed to collaborate with us to
run PALS on his apparatus and provide expert analysis.
Modeling:
Our goal is to complete molecular modeling of
polymer/inorganic nanocomposite and gas transport therein. Current
theories for increased permeability in glassy polymer nanocomposites hinge on
interfacial effects and inhibited polymer packing. Therefore, we are particularly interested in the interface
between the polymer and the nanoparticle, as well as
the difference in polymer configuration due to the presence of nanoparticles.
We have set up the using the commercial molecular modeling software Materials
Studio (Accelrys) on a computational cluster to drastically decrease the
computational time for each run and are now working to set up the MS Discover
Parallel. We successfully modeled PDMS by running molecular dynamics and a
series of NVT/NPT equilibration steps to obtain the equilibrium structure. We
have simulated gas diffusion and are working to determine free volume in the
cell.
Research Students:
In the second year of this award, eight students majoring in
chemistry and engineering participated in the project. The students were
trained on the theory and use of the SEM, learned techniques and developed
protocols for solution-casting polymer membranes, synthesized Stšber particles,
learned theory and set up a system for PALS, written literature reviews, given
technical presentations, presented a poster at the Gordon Research Conference
on Membranes: Materials and Processes, and toured local chemical companies,
including Chevron and Amgen. The
students are listed below, with their status as of August 2008 given in
parentheses: