

47099-AC5
Hydrogenation Reactions on Palladium Involving Subsurface Hydride
We have
been utilizing low-temperature, ultrahigh vacuum scanning tunneling microscopy
(
We have
observed that both the surface preparation and the temperature at which
thiophene is deposited affects adsorption.
Figure 1 shows
We acquired
action spectroscopy over individual thiophene molecules to address their mobility
at 4 K. Figure 2 shows A) a representative
action spectrum along with a sequence of
We have recorded initial differential conductance spectroscopy (dI/dV) over both the Pd{111} surface (Figure 3A) and single thiophene molecules (Figure 3B) in order to characterize the electronic structure of the hydrogenation system prior to reaction. Although we observe effective quenching of the Pd surface state by thiophene adsorption, we will continue optimizing spectroscopic parameters in order to obtain reproducible and interpretable single-molecule vibrational spectroscopy results.
FIGURES Figures
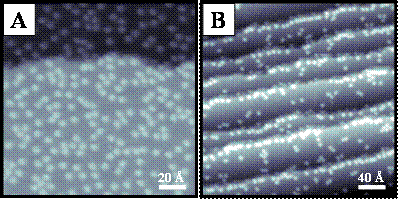
Figure 1. A) STM image (143 Å × 143 Å, Vs = 0.1 V, It = 150 pA) of molecular thiophene adsorbed on a Pd{111} surface at 4 K. Dosed after the Pd surface was allowed to reach 4 K, thiophene appears randomly distributed on the surface. The two regions seen in the image are atomic terraces separated by a single Pd atomic step edge. B) STM image (286 Å × 286 Å, Vs = -0.3 V, It = 100 pA) of molecular thiophene aligned preferentially along the tops and bottoms of step edges on a Pd{111} surface at 4 K. Thiophene was dosed at ca. 20 ‑ 40 K and thus had some surface mobility on adsorption.
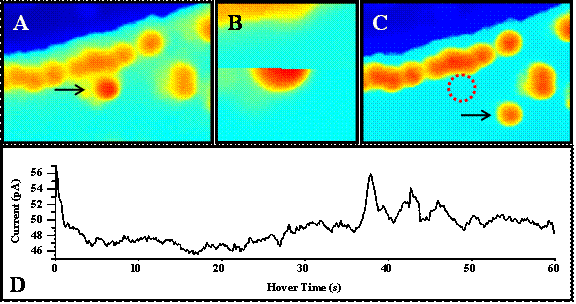
Figure
2. A) STM image (57 Å × 38 Å, Vs = -0.2 V, It = 100 pA) of molecular
thiophene adsorbed near a Pd{111} step edge at 4 K. The arrow denotes a single molecule over
which action spectroscopy is performed.
B) STM image (14 Å × 14 Å, Vs
= -0.2 V, It = 100 pA)
acquired before and after action spectroscopy.
The thiophene moved out of the frame of imaging during spectroscopy,
resulting in what appears to be a partially imaged molecule. C) STM image (57 Å × 38 Å, Vs =-0.2 V, It = 100 pA) after
action spectroscopy. This STM image shows
the new location of the molecule from A.
D) I vs. t spectrum (Vgap
= -0.35 V, Igap = 100 pA)
acquired over 60 seconds exhibits motion of the molecule under the STM tip
before finally moving out of the frame in B.
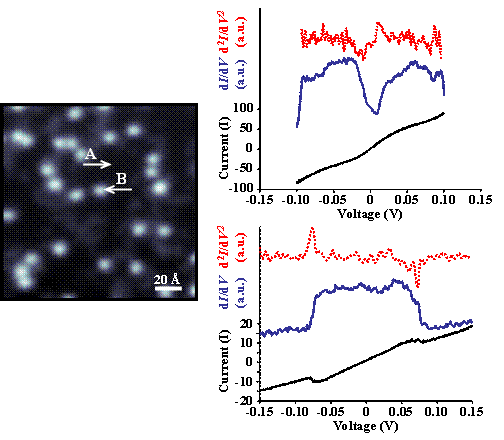
Figure 3. STM image (143 Å × 143 Å, Vs = -0.45 V, It = 40 pA) of molecular thiophene adsorbed on Pd{111} at 4 K and accompanying spectra acquired over A) Pd and B) thiophene (Vgap = -0.01 V, Igap = 40 pA). The broken red traces are the result of mathematically differentiating the dI/dV spectra (blue). The I/V response and subsequently the dI/dV response of atomic Pd and molecular thiophene are markedly different. A notable feature is the apparent quenching of the surface state about the Fermi energy by the adsorbate. Once parameters are optimized for IETS, single-molecule vibrational spectra will be acquired simultaneously, enabling the assignment of vibrational modes.