45004-B10
Investigating the Chemistry of Boranes with Single-Walled Carbon Nanotubes Using FTIR and Raman Spectroscopies
This year, the project has made slow but steady progress. Work in the first year of the grant found evidence that borane complexes did not react with single-walled carbon nanotubes (SWCNTs) at 0°C or at room temperature. However, research in fall 2007 found that, when irradiated with ultraviolet light, SWCNTs do appear to react with borane-tetrahydrofuran. Continuation of this work in summer 2008 found that SWCNTs appear to react with borane-dimethyl sulfide but not with borane-triethylamine or borane-tert-butylamine when irradiated with ultraviolet light. These results suggest that the more reactive borane complexes will react with SWCNTs when irradiated with ultraviolet light, but the more stable borane complexes will not. The reaction appears to be a hydroboration reaction, which attaches a –BH2 group and a –H group to the nanotubes. Efforts to isolate these intermediates were difficult, presumably because of the reactivity of the –BH2 group. Nonetheless, one of the student researchers, through careful experimentation, did reproducibly obtain infrared spectra of SWCNTs with peaks at 2300 cm–1 and 2900 cm–1 attributed to B-H stretches and C-H stretches, respectively, as shown in Figure 1.
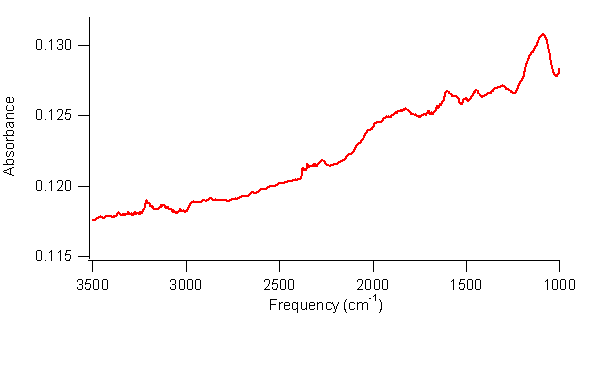
Figure
1. FTIR spectrum of SWCNTs reacted with borane-tetrahydrofuran complex under UV irradiation. Peaks at 2300 cm–1 are consistent
with B–H stretches, and peaks at 2950 cm–1 are consistent with C–H
stretches. Work in spring 2008 determined that the reaction is
initiated by light of wavelength less than 300 nm. This suggests that the SWCNTs are absorbing
the ultraviolet light to undergo a p→ p* transition. This could interrupt the aromaticity
of the nanotubes' structure, increasing their
reactivity. Computational studies
indicated that the hydroboration reaction is endoergonic, suggesting that it is likely not spontaneous
without some energy input. Currently,
experiments are under way to elucidate the photochemical reaction mechanism.
To further test our results, in late summer 2008 and in fall
2008, SWCNTs reacted with borane-tetrahydrofuran
under ultraviolet irradiation were then reacted with a sodium
hydroxide/hydrogen peroxide mixture.
These conditions are those of a classic hydroboration-oxidation
reaction, in which borane adds across a double bond
to form –BH2 and –H groups, and the –BH2 group is
subsequently oxidized to –OH. Infrared
spectra of the SWCNTs subjected to these steps show O–H stretches at 3400 cm–1
and C–H stretching peaks at 2900 cm–1, as shown in Figure 2, which
are consistent with our hypothesis. This
strongly suggests that the SWCNTs are undergoing a photo-initiated hydroboration/oxidation reaction.
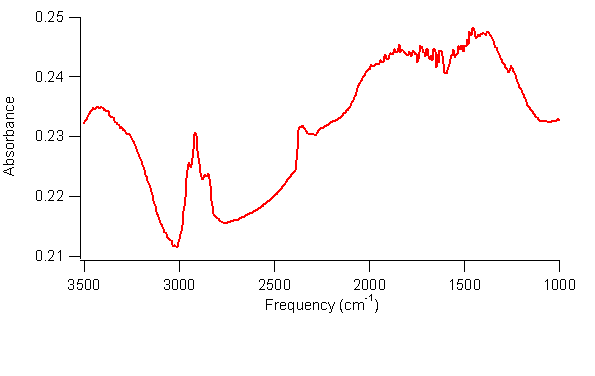
Figure
2. FTIR spectrum of hydroborated and oxidized SWCNTs. The broad peak at 3400 cm–1 is
consistent with an O–H stretch, and peaks at 2900 cm–1 are
consistent with C–H stretches. The peak at 2300 cm–1 might be unreacted –BH2 on the SWCNTs. A second project is investigating the reductive amination of SWCNTs.
Certain borane complexes, such as a-picoline borane, have been shown to allow for the reduction of
carbonyl groups, followed by an amine attachment. Because SWCNTs can easily be oxidized to yield
carboxylic acid functional groups, reductive amination
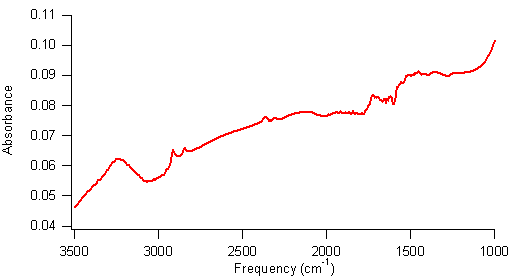
could potentially be a new pathway to producing functionalized SWCNTs. Figure 3 shows an FTIR spectrum of carboxylated SWCNTs that have undergone reductive amination with a-picoline borane and butylamine. The
peaks just below 3000 cm–1 are consistent with the sp3-hybridized
C-H stretches of butylamine molecules. The broad peak at 3250 cm–1 is
consistent with an amide that could form during the reductive amination, although it is broader than expected. This spectrum suggests that the reductive amination reaction might be successful and is worthy of further
research.
Figure 3. FTIR
spectrum of carboxylated SWCNTs that have undergone a reductive amination. The peak at 3250 cm–1 might be an
amide peak, and peaks at 2900 cm–1 are C–H stretches.
The peak at 1700 cm–1 is unreacted carbonyl groups. In summary, we are close to demonstrating that photochemical
hydroboration/oxidation can functionalize
SWCNTs. Additionally, a reductive amination reaction involving a borane
complex also appears to have promise as a means of functionalizing carboxylated SWCNTs.
Work on these two projects will continue.