Back to Table of Contents
46565-AC5
A Transport Theory, Molecular Dynamics Simulations and Experiments on the Adsorption of Surfactant From Micellar Solutions to an Initially Clean Air/Water Interface
Charles Maldarelli, City College of New York
This research program examines
the mechanisms by which surfactants
adsorb from an aqueous solution onto an initially clean air/water
interface. This study focuses particularly on adsorption from solutions in which
the concentration of surfactant in the aqueous phase, Cbulk, is
above the critical concentration (CCMC) for which aggregates
(micelles) form in the bulk. When a clean interface forms in a solution with Cbulk<CCMC,
un-aggregated surfactant molecules diffuse towards the interface, and then
kinetically adsorb onto the surface. When surfactant adsorbs from a micellar
solution to a clean interface, measurements of the reduction in tension have
demonstrated that the presence of micelles accelerates the adsorption process,
with the rate of tension reduction increasing with Cbulk. One mechanism for this acceleration is
that un-aggregated surfactant adsorbs onto the interface. This disturbs the
un-aggregated surfactant -- aggregate equilibrium, causing aggregates to
disassemble, which replenishes the free surfactant and accelerates the overall
adsorption process.
We have
obtained evidence that micelles can directly adsorb onto the surface and
release surfactant to populate the surface, studying the polyethoxylated
surfactant C
14E
6 (CH
3(CH
2)
13(OCH
2CH
2)
6OH) which forms approximately spherical
aggregates. We have used a small, hydrophobic molecule, the dye Nile Red, to
track the micelle transport. Nile Red has a very low solubility in water; when
dissolved in an aqueous phase with surfactant aggregates, the molecule
partitions almost exclusively into the hydrophobic interior of the aggregate.
In aqueous solutions without surfactant, Nile Red, at concentrations below its
solubility limit, shows no tendency to adsorb onto a surface; however in the
presence of a surface monolayer the hydrophobic part of the monolayer acts as a
host for the Nile Red, and the dye adsorbs to the surface and decreases the
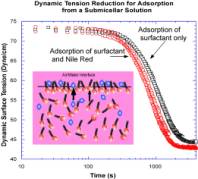
tension. In the figure the reduction in tension, using the pendant bubble
method, as surfactant adsorbs to a clean interface from a submicellar 0.17 C
CMC
C
14E
6 solution and a 0.17 C
CMC C
14E
6
solution with 0.2 micrograms/ml Nile Red (below its solubility limit). After an
induction time necessary for the formation of the monolayer, the Nile Red
adsorbs to the surface and reduces the
tension relative to the
tension recorded without Nile Red. The adsorption of both Nile Red and
surfactant results in a mixed monolayer with an equilibrium tension
approximately 2.5 dyne/cm lower than the tension without Nile Red,
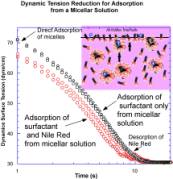
Plotted are
relaxations for adsorption to a clean interface from a micellar, 15 C
solution with the same
concentration of Nile Red as in the sub-micellar solution experiments (0.2
micrograms/ml, resulting in one dye molecule for every two micelle aggregates ). The adsorption of surfactant is much faster than
in the case of a sub-micellar solution, and the induction period for monolayer
formation is less than a second. The data indicates that after one second, the
amount of dye on the surface is already very large, with a difference in
tensions of approximately 5-7 dyne/cm after one second from the formation of
the interface. This is double the largest difference obtained in the
sub-micellar relaxations. Direct adsorption of micelles during the induction
period can account for this large difference in tensions, with the adsorbed
micelle then breaking up and releasing monomer as well as the incorporated Nile
Red onto the surface. If the Nile Red instead adsorbed to the surface only by
disassembly of the bulk micelles followed by adsorption of the released dye,
the tension after one second between the micellar solutions with and without
Nile Red would not be very different. This is because adsorption of Nile Red is
small during the induction period, and free dye resulting from micelle
disassembly is more likely to
partition into bulk micelles in their vicinity. The large difference in
tension, in fact, represents an overshoot. The excess transports back into the
bulk as the Nile Red on the surface desorbs and becomes sequestered back into
the micelles in the bulk. This desorption is accompanied by an increase in
tension, followed by a reduction as the more surface active polyethoxylate
adsorbs to equilibrium.