46070-AC7
Design and Synthesis of Liquid Crystalline Polymer Brush Films
Our project involves the synthesis of liquid crystalline monomers and chiral moities that will be incorporated into side chain polymers. Furthermore, the polymers will be synthesized by a grafting-from approach from silicon oxide substrates. The polymer brush films should exhibit liquid crystalline behavior, particularly the chiral nematic, or so-called cholesteric phase. These LC brushes are expected to respond to external stimuli, such as the binding of a guest molecule.
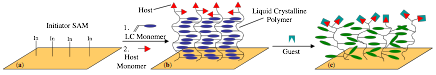
Figure 1 illustrates our strategy for creating a host-guest LCP brush film that could respond to the binding of a guest molecule. First, a SAM is deposited onto a substrate such that the terminal group is a controlled free radical initiator (ATRP or RAFT). This substrate is then used to synthesize an LCP brush by the grafting-from (GF) technique, which is capped by a short block containing a host monomer. Previously LCP brushes exhibited LC textures, which were visible by polarized optical microscopy in films less than 40 nm. The resulting LCP brush (Figure 1b) could be aligned to form a homogeneous planar film by shear alignment or by surface rubbing; homeotropic alignment could be imparted by modifying the SAM or other surface treatments. Furthermore, a ligand could be incorporated into the brush so that it will bind to a specific analyte, such as a metal ion or biological molecule. Upon binding of the guest molecule, the surface of the brush is perturbed and this causes a realignment of the bulk film, resulting in a change in color and/or texture (Figure 1c). Since polymer chains may respond slowly, these brush films could be swelled with low molar mass LCs to increase the amplification of the surface perturbations.
Figure 1. Design strategy for an end-capped liquid crystalline polymer brush sensor: (a) Controlled free radical initiator is chemisorbed to substrate by a self-assembled monolayer; (b) ATRP or RAFT polymerization is initiated in the presence of an LC monomer to create an LC brush. (c) A host monomer is added to synthesize a short block that caps the LCP brush; and (d) The guest molecule binds to the host and perturbs the interface, the disorder is propagated to the bulk film and results in a change in texture or color. (Note: a random copolymer could also be synthesized where the host is distributed throughout the chain; this would be easier than a controlled polymerization.)
The first stage of this program involves the design of monomers based on known examples of LC side chain polymers. Specifically, we are focusing on nematic LCPs that have transition temperatures near room temperature. These polymers will be tethered to glass, silicon, and quartz substrates via surface initiated polymerization techniques. In particular, we will utilize GF free radical initiators that have been described in the literature and previously synthesized in our laboratory (see below). The second stage of the project will involve a thorough characterization of the LC properties of the polymer brushes. We will align the brushes and compare the properties of the tethered brush to that of an untethered LCP, which is merely physisorbed to the substrate. The third stage will involve the incorporation of functional groups (hosts) that will respond to a change in pH by altering the texture after the LCP brush is immersed into an aqueous solution. In the fourth stage we will incorporate chiral units into the LCP in order to create a film that reflects visible light. The fifth stage will examine the switching properties of these cholesteric brushes after adding hosts specific for metal ions and the protein avidin. The response of these films will be monitored by POM, UV-vis, FT-IR, and ellipsometry as a function of time, temperature, and analyte concentration.
Currently we are in the initial stages of the project and have succeeded in synthesizing a relatively large amount of our first monomer (7), which is described in Scheme 1a. The proton NMR spectrum is displayed in Figure 2 and confirms the synthesis. In addition, we have nearly completed the synthesis of our first chiral monomer (10) as illustrated in Scheme 1b. We are now in the process of synthesizing random copolymers of the two monomers and examining the LC properties of the polymers. Our fist experiments will be in the bulk and then we will initiate polymerizations from quartz substrates.
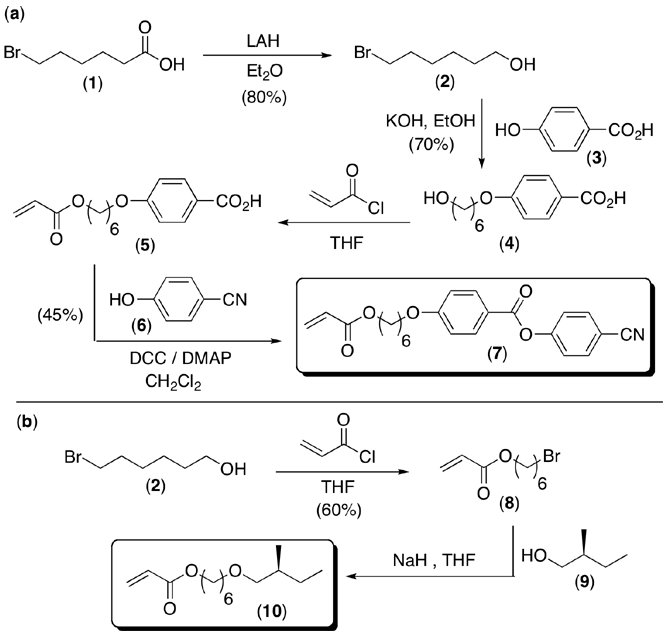
Scheme 1 (a) Synthesis of liquid crystalline monomers and (b) chiral side-chain moieties for incorporation into random copolymer brushes.
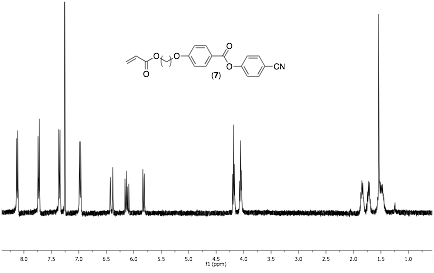
Figure 2. 1H NMR spectrum of acrylate monomer 7.